Summary
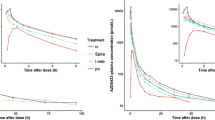
Budesonide, a topically potent glucocorticoid, was administered to 4 healthy volunteers by i.v. infusion and by nasal instillation of 100 µg tritium-labelled drug. Plasma was analyzed by liquid chromatography plus scintillation counting of collected fractions. After i.v. administration the plasma clearance was 0.92 l/min and the apparent volume of distribution was 2.8 l/kg. After nasal administration, the time to reach the peak plasma level was approximately 30 min, and the systemic availability was 102%. Budesonide had marginal effects on plasma cortisol and white blood cell counts either after i.v. or nasal administration. Thus, nasally instilled budesonide in solution is rapidly and completely absorbed from the nasal mucosa. The systemic effects after this clinically recommended nasal dose were negligible.
Similar content being viewed by others
References
Ellul-Micallef R, Johansson SÅ (1980) Budesonide: A new corticosteroid in bronchial asthma. Eur J Respir Dis 61: 167–173
Pipkorn U, Rundcrantz H, Lindqvist N (1980) Budesonide — a new nasal steroid. Rhinology 18: 171–175
Pipkorn U, Rundcrantz H (1982) Budesonide and beclomethasone dipropionate in hay fever — a single-blind comparison. Eur J Respir Dis 63 [Suppl 122]: 221–230
Pipkorn U, Geterud Å (1984) A comparative trial testing budesonide and flunisolide nasal sprays in patients with seasonal allergic rhinitis. Ann Allergy 52: 183–186
Pipkorn U (1982) Budesonide and nasal allergen challenge testing in man. Allergy 37: 129–134
Ryrfeldt Å, Andersson P, Edsbäcker S, Tönnesson M, Davies D, Pauwels R (1982) Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur J Respir Dis 63 [Suppl 122]: 86–95
Edsbäcker S, Jönsson S, Lindberg C, Ryrfeldt Å, Thalén A (1983) Metabolic pathways of the topical glucocorticoid budesonide in man. Drug Metab Dispos 11 (6): 590–596
Dahlberg E., Thalén A, Brattsand R, Gustafsson JÅ, Johansson U, Roempke K, Saartok T (1984) Correlation between chemical structure, receptor binding and biological activity of some novel highly active 16α, 17α-acetal substituted glucocorticoids. Mol Pharmacol 25: 70–78
Hartiala J (1976) Steroid metabolism in adult lung. Agents Actions 6/4: 522–526
Andersson P, Ryrfeldt Å (1984) Biotransformation of the topical glucocorticoids budesonide and beclomethasone 17 α, 21-dipropionate in human liver and lung homogenate. J Pharm Pharmacol 36: 763–765
Brittebo EB (1982) Metabolism of progesterone by the nasal mucosa in mice and rats. Acta Pharmacol Toxicol 51: 441–445
Andersson P, Edsbäcker S, Ryrfeldt Å, von Bahr C (1982) In vitro biotransformation of glucocorticoids in liver and skin homogenate fraction from man, rat and hairless mouse. J Steroid Biochem 16: 787–795
Ryrfeldt Å, Edsbäcker S, Pauwels R (1984) Kinetics of the epimeric glucocorticoid budesonide. Clin Pharmacol Ther 35: 525–530
Brittebo EB, Rafter JJ (1984) Steroid metabolism by rat nasal mucosa; studies on progesterone and testosterone. J Steroid Biochem 20: 1147–1151
Chaplin MD, Cooper WC, Segre EJ, Dren J, Jones RE, Nerenberg C (1980) Correlation of flunisolide plasma levels to eosinopenic response in humans. J Allergy Clin Immunol 65 [6]: 445–453
Wilkinson GR, Shand DG (1975) A physiological approach to hepatic drug clearance. Clin Pharmacol Ther 18 [4]: 377–390
Nies AS, Shand DG, Wilkinson GR (1976) Altered hepatic blood flow and drug disposition. Clin Pharmacokinet 1: 135–155
Bergrem H, Grøttum P, Rugstad HE (1983) Pharmacokinetics and protein binding of prednisolone after oral and intravenous administration. Eur J Clin Pharmacol 24: 415–419
Legler UF, Frey FJ, Benetz LZ (1982) Prednisolone clearance at steady state in man. J Clin Endocrinol Metab 55: 762–767
Loo JCK, McGilveray IJ, Jordan N, Moffat J, Brien R (1978) Dose dependent pharmacokinetics of prednisone and prednisolone in man. J Pharm Pharmacol 30: 736
McAllister WAC, Wintfield CR, Collins JV (1981) Pharmacokinetics of prednisolone in normal and asthmatic subjects in relation to dose. Eur J Clin Pharmacol 20: 141–145
Meffin PJ, Brooks PM, Sallustio BC (1984) Alterations in prednisolone disposition as a result of time of administration, gender and dose. Br J Clin Pharmacol 17: 395–404
Pickup ME, Lowe JR, Leatham PA, Rhind VM, Wright V, Downie WW (1977) Dose dependent pharmacokinetics of prednisolone. Eur J Clin Pharmacol 12: 213–219
Rose JQ, Yurchak AM, Jusko WJ (1981) Dose dependent pharmacokinetics of prednisone and prednisolone in man. J Pharmacokinet Biopharm 9: 389–416
Tanner A, Bochner F, Caffin J, Halliday J, Powell L (1979) Dose dependent prednisolone kinetics. Clin Pharmacol Ther 25: 571–578
Mygind N (1982) Topical steroid treatment for allergic rhinitis and allied conditions. Clin Otolaryngol 7: 343–352
Johansson SÅ, Andersson KE, Brattsand R, Gruvstad E, Hedner P (1982) Topical and systemic glucocorticoid potencies of budesonide and beclomethasone dipropionate in man. Eur J Clin Pharmacol 22: 523–529
Newman SP, Pavia D, Morén F, Sheahan NF, Clarke SW (1981) Deposition of pressurised aerosols in the human respiratory tract. Thorax 36: 52–55
Harris DM (1975) Some properties of beclomethasone dipropionate and related steroids in man. Postgrad Med J 51 [Suppl 4]: 20–25
Balle VÅ, Pedersen V, Engby B (1982) The treatment of perennial rhinitis with a new non-halogenated, topical, aerosol packed steroid, budesonide. Acta Otolaryngol 94: 169–173
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Edsbäcker, S., Andersson, K.E. & Ryrfeldt, Å. Nasal bioavailability and systemic effects of the glucocorticoid budesonide in man. Eur J Clin Pharmacol 29, 477–481 (1985). https://doi.org/10.1007/BF00613465
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00613465