Summary
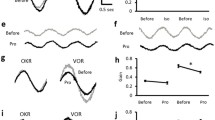
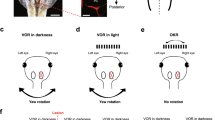
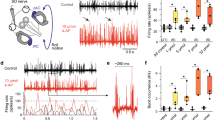
Noradrenaline (NA) has been implicated as a neuromodulator in plasticity, presumably facilitating adaptive processes. Recent experiments by others have suggested a modulatory role of NA in adaptive changes in the vestibulo-ocular reflex (VOR). These experiments showed that general depletion of brain NA resulted in a decreased ability to produce adaptive changes in the VOR gain. In order to identify the specific brain region responsible for these effects, as well as the nature of the adrenoceptors involved, we injectedβ-adrenergic substances bilaterally into the flocculus of rabbits. The flocculus is known to receive noradrenergic afferents and, moreover, ablation of the flocculus interferes strongly with the normal adaptive changes in the VOR gain. We injected theβ-agonist isoproterenol and theβ-antagonist sotalol, and compared the adaptive capacity of the rabbits after these injections to that in a situation without injection. The rabbit was oscillated in a direction opposite to the direction of motion of the platform on which the rabbit was mounted, a condition which normally results in an increase in the VOR gain, measured either in light or in darkness. Injection of theβ-agonist did not greatly affect the adaptation of the VOR measured in the light. In darkness, the increase in gain after the injection of isoproterenol was larger than in the non-injection experiments in 9 out of 10 rabbits. Theβ-antagonist sotalol reduced the adaptation of the VOR gain significantly in the light, as well as in darkness. In a control condition without pressure for adaptation (only intermittent testing of the VOR gain over a period of 2.5 h), the gain of the VOR either remained unaffected or was only slightly affected by similar injections ofβ-adrenergic agents in individual rabbits. For the group as a whole, these effects were insignificant. We conclude from these results that noradrenergic systems facilitate the adaptation of the VOR gain to retinal slip in rabbits, without affecting the VOR gain directly. At least part of this influence is exerted throughβ-receptors located in the cerebellar flocculus.
Similar content being viewed by others
References
Abercrombie ED, Jacobs BL (1987) Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressfull and nonstressfull stimuli. J Neurosci 7:2837–2843
Albus JS (1971) A theory of cerebellar function. Math Biosci 10:25–61
Berthoz A, Melvill Jones G (1985) Adaptive mechanisms in gaze control: facts and theories. Reviews of oculomotor research, Vol 1. Elsevier, Amsterdam
Bloom FE, Hoffer BJ, Siggins GR (1971) Studies on norepinephrine-containing afferents to Purkinje cells of rat cerebellum. I. Localization of the fibers and their synapses. Brain Res 25:501–521
Bylund DB, U'Prichard DC (1983) Characterization of α1 and α2-adrenergic receptors. Int Rev Neurobiol 24:343–431
Collewijn H (1977) Eye- and head movements in freely moving rabbits. J Physiol (Lond) 266:471–498
Collewijn H, Grootendorst AF (1978) Adaptation of the rabbit's vestibulo-ocular reflex to modified visual input: importance of stimulus conditions. Arch Ital Biol 116:273–280
Collewijn H, Grootendorst AF (1979) Adaptation of optokinetic and vestibulo-ocular reflexes to modified visual input in the rabbit. In: Granit R, Pompeiano O (eds) Reflex control of posture and movement. Elsevier, Amsterdam, pp 771–781
Collewijn H, Van der Steen J (1987) Visual control of the vestibulo-ocular reflex in the rabbit: a multi-level interaction. In: Glickstein M, Yeo C, Stein J (eds) Cerebellum and neuronal plasticity. NATO ASI Series A, Vol 148. Plenum, Press, New York, pp 277–291
d'Ascanio P, Bettini E, Pompeiano O (1985) Tonic inhibitory influences of locus coeruleus on the response gain of limb extensors to sinusoidal labyrinth and neck stimulations. Arch Ital Biol 123:69–100
d'Ascanio P, Bettini E, Pompeiano O (1989) Inhibition of vestibulospinal reflexes during the episodes of postural atonia induced by unilateral lesion of the locus coeruleus in the decerebrate cat. Arch Ital Biol 127:81–97
Dietrichs E (1988) Cerebellar cortical and nuclear afferents from the feline locus coeruleus complex. Neuroscience 27:77–92
Edwards SB, Hendrickson A (1981) Neuroanatomical tracttracing methods. Plenum Press, New York
Foote SL, Bloom FE, Aston-Jones G (1983) Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63:844–914
Fuxe K (1965) Evidence for the existence of monoamine neurons in the central nervous system. IV. The distribution of monoamine terminals in the central nervous system. Acta Physiol Scand [Suppl] 247:37–85
Gilbert P (1975) How the cerebellum could memorise movements. Nature 254:688–689
Gordon B, Moran J, Trombley P, Soyke J (1986) Visual behaviour of monocularly deprived kittens treated with 6-hydroxydopamine. Brain Res 389:21–29
Graf W, Simpson JI, Leonard CS (1988) The spatial organization of visual messages to the flocculus of the rabbit's cerebellum. II. Complex and simple spike responses of Purkinje cells. J Neurophysiol 60:2091–2121
Greenshaw AJ (1985) Electrical and chemical stimulation of brain tissue in vivo. In: Boulton AA, Baker GB (eds) General pneurochemical techniques: neuromethods (1). Humana Press, Cliton, pp 233–277
Ito M (1984) The cerebellum and neural control. Raven Press, New York.
Ito M, Shiida T, Yagi N, Yamamoto M (1974a) The cerebellar modification of rabbit's horizontal vestibulo-ocular reflex induced by sustained head rotation combined with visual stimulation. Proc Jpn Acad 50:85–89
Ito M, Shiida T, Yagi N, Yamamoto M (1974b) Visual influence on rabbit horizontal vestibulo-ocular reflex presumably effected via the cerebellar flocculus. Brain Res 65:170–174
Ito M, Jastreboff PJ, Miyashita Y (1979) Adaptive modification of the rabbit's horizontal vestibulo-ocular reflex during sustained vestibular and optokinetic stimulation. Exp Brain Res 37:17–30
Ito M, Jastreboff PJ, Miyashita Y (1982) Specific effects of unilateral lesions in the flocculus upon eye movements in albino rabbits. Exp Brain Res 45:233–242
Kasamatsu T (1987) Norepinephrine hypothesis for visual cortical plasticity: thesis, antithesis, and recent development. Curr Top Dev Biol 21:367–389
Kasamatsu T, Pettigrew DJ (1976) Depletion of brain catecholamines: failure of ocular dominance shift after monocular occlusion in kittens. Science 194:202–209
Kasamatsu T, Pettigrew JD, Ary M (1979) Restoration of visual cortical plasticity by local microperfusion of norepinephrine J Comp Neurol 185:163–182
Kebabian JW, Zatz M, Romero JA, Axelrod J (1975) Rapid changes in rat pinealβ-adrenergic receptor: alteration in 1-[3H] alprenolol binding and adenylate cyclase. Proc Natl Acad Sci USA 72:3735–3739
Keller EL, Smith MJ (1983) Suppressed visual adaptation of the vestibulo-ocular reflex in catecholamine depleted cats. Brain Res 258:323–327
Kimoto Y, Satoh K, Sakumoto T, Tohyama M, Shimizu N (1978) Afferent fiber connections from the lower brain stem to the rat cerebellum by the horseradish peroxidase method combined with MAO staining, with special reference to noradrenergic neurons. J Hirnforsch 19:85–100
Kimoto Y, Tohyama M, Satoh K, Sakumoto T, Takahashi Y, Shimizu N (1981) Fine structure of rat cerebellar noradrenaline terminals as visualized by potassium permanganate ‘in situ’: perfusion, fixation and method. Neuroscience 6:47–58
Langer T, Fuchs AF, Scudder CA, Chubb MC (1985) Afferents to the flocculus of the cerebellum in the rhesus macaque as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol 235:1–25
Lorton D, Davis JN (1987) The distribution ofβ 1 andβ 2-adrenergic receptors of normal and reeler mouse brain: an in vitro autoradiographic study. Neuroscience 23:199–210
Marr D (1969) A theory of cerebellar cortex. J Physiol (Lond) 202:437–470
McElligot JG, Freedman W (1988a) Vestibulo-ocular reflex adaptation in cats before and after depletion of norepinephrine. Exp Brain Res 69:509–521
McElligot JG, Freedman W (1988b) Central and cerebellar norepinephrine depletion and vestibulo-ocular reflex (VOR) adaptation. In: Flohr H (eds) Post-lesion neuronal plasticity. Springer, Berlin Heidelberg New York Tokyo, pp 661–674
Miles FA, Lisberger SG (1981) Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annu Rev Neurosci 4:273–299
Minneman KP, Hegstrand LR, Molinoff PB (1979) Simultaneous determination ofβ 1 andβ 2-adrenergic receptors in tissues containing both receptor types. Mol Pharmacol 16:34–46
Minneman KP, Pittman RN, Molinoff PB (1981)β-adrenergic receptor subtypes: properties, distribution and regulation. Annu Rev Neurosci 4:419–461
Miyashita Y, Watanabe E (1984) Loss of vision-guided adaptation of the vestibulo-ocular reflex after depletion of brain serotonin in the rabbit. Neurosci Lett 51:177–182
Myers RD (1966) Injection of solutions into cerebral tissue: relation between volume and diffusion. Physiol Behav 1:171–174
Nagao S (1983) Effect of vestibulocerebellar lesions upon dynamic characteristics and adaptation of vestibulo-ocular and optokinetic responses in pigmented rabbits. Exp Brain Res 53:36–46
Olson L, Fuxe K (1971) On the projections from the locus coeruleus noradrenaline neurons: the cerebellar innervation. Brain Res 28:165–171
Palacios JM, Kuhar MJ (1980)β-adrenergic receptor localization by light microscopic autoradiography. Science 208:1378–1380
Palacios JM, Kuhar MJ (1982)β-adrenergic receptor localization in rat brain by light microscopic autoradiography. Neurochem Int 4:473–490
Pompeiano M, Galbani P, Ronca-Testoni S (1989) Distribution ofβ-adrenergic receptors in different cortical and nuclear regions of cat cerebellum, as revealed by binding studies. Arch Ital Biol 127:115–132
Pompeiano O (1988) Basic aspects of vestibulospinal function. In: Igarashi M, Nute KG (eds) Proceedings symposium on vestibular organs and altered force environment. NASA Space Biochemical Research Institute, Houston, pp 1–23
Pompeiano O, d'Ascanio P, Horn E, Stampacchia G (1987) Effects of local injection of theα 2-adrenergic agonist clonidine into the locus coeruleus complex on the gain of vestibulospinal and cervicospinal reflexes in decerebrate cats. Arch Ital Biol 125:225–269
Pompeiano O, Van Neerven J, Van Der Steen J, Collewijn H (1988) Effects of injection of noradrenergic and GABA-ergic substances in the flocculus on the gain of the VOR and OKN in the rabbit. Eur J Neurosci [Suppl] 1:6.2
Rainbow TC, Parsons B, Wolfe BB (1984) Quantitative autoradiography ofβ 1 andβ 2-adrenergic receptors in rat brain. Proc Natl Acad Sci USA 81:1585–1589
Schairer JO, Bennett MVL (1986a) Changes in gain of the vestibulo-ocular reflex induced by combined visual and vestibular stimulation in goldfish. Brain Res 373:164–176
Schairer JO, Bennett MVL (1986b) Changes in gain of the vestibulo-ocular reflex induced by sinusoidal visual stimulation in goldfish. Brain Res 373:177–181
Segal M, Pickel J, Bloom FE (1973) The projection of the nucleus locus coeruleus: an autoradiographic study. Life Sci 13:817–821
Silberman EK, Vivaldi E, Garfield J, McCarley RW, Hobson JA (1960) Carbachol triggering of desynchronized sleep phenomena: enhancement via small volume infusions. Brain Res 191:215–224
Simpson JI, Graf W, Leonard C (1981) The coordinate system of visual climbing fibers to the flocculus. In: Fuchs A, Becker W (eds) Progress in oculomotor research: developments in neuroscience, Vol 12. Elsevier, Amsterdam, pp 475–484
Somana R, Walberg F (1978) The cerebellar projection from locus coeruleus as studied with retrograde transport of horseradish peroxidase in the cat. Anat Embryol 155:87–94
Stampacchia G, d'Ascanio P, Horn E, Pompeiano O (1988) Gain regulation of the vestibulospinal reflex following microinjection of aβ-adrenergic agonist or antagonist into the locus coeruleus and the dorsal pontine reticular formation. Adv Otorhinolaryngol 41:134–141
Sutin J, Minneman KP (1985) Adrenergicβ-receptors are not uniformly distributed in the cerebellar cortex. J Comp Neurol 236:547–554
Waterhouse BD, Sessler FM, Cheng JT, Woodward JD, Azizi SA, Moises HC (1988) New evidence for a gating action of norepinephrine in central neuronal circuits of mammalian brain. Brain Res Bull 21:425–432
Watson M, McElligott JG (1983) 6-OHDA induced effects upon the acquisition and performance of specific locomotor tasks in rats. Pharmacol Biochem Behav 18:927–934
Watson M, McElligott JG (1984) Cerebellar norepinephrine depletion and impaired acquisition of specific locomotor tasks in rats. Brain Res 296:129–138
Yamamoto Y, Ishikawa M, Tanaka C (1977) Catacholaminergic terminals in the developing and adult rat cerebellum. Brain Res 132:355–361
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Neerven, J., Pompeiano, O., Collewijn, H. et al. Injections of β-noradrenergic substances in the flocculus of rabbits affect adaptation of the VOR gain. Exp Brain Res 79, 249–260 (1990). https://doi.org/10.1007/BF00608233
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00608233