Summary
-
1.
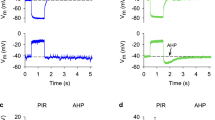
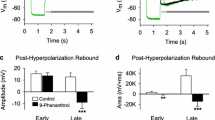
Current-clamp and voltage-clamp experiments were performed on L-neurones in the median ocellar nerve of locusts (Locusta migratoria). Constant-current steps applied at the normal resting potential (which ranges around −40 mV) elicited electrotonic potentials that displayed outward and inward rectification. Positive constant-current steps superimposed to a negative holding current elicited small-amplitude action potentials whose threshold was around −65 mV.
-
2.
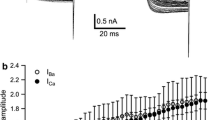
Under voltage-clamp two fast currents were observed. A fast inward current reflecting the action potentials in the current clamp experiments had a threshold of −65 mV. It could be blocked by TTX and its voltage characteristics were similar to those of I(Na) in giant cockroach axons. The steady-state inactivation curve shows that the channels of this current are completely inactivated at the L-neurones' resting potential.
-
3.
A fast outward current partially shunting the fast inward current could be blocked with 4-AP. Its threshold was at about −50 mV, and at −40 mV about 20% of this current could be activated by depolarizing steps. The reversal potential was around −75 mV and the voltage characteristics were very similar to those of a transient potassium current (I(A)) inDrosophila flight muscle.
-
4.
Additionally slowly activating time- and voltage-dependent currents were observed under voltage-clamp. These currents probably reflected the existence of further voltage-gated channels but the possibility of synaptically mediated conductance changes could not be excluded.
-
5.
The results demonstrate that locust L-neurones are capable of generating action potentials of small amplitude. The currents underlying these action potentials are similar to those described in spiking neurones. Their voltage dependence shows that the ion channels of these currents are mostly in the inactivated state at the normal resting potential of the L-neurones. Inactivation can be removed by shifting the membrane to a more negative level. Possible reasons for the low resting potential, which is mainly responsible for the ‘nonspiking’ state of an L-neurone, are discussed.
Similar content being viewed by others
Abbreviations
- L-neurone :
-
large axoned ocellar neurone
- S-neurone :
-
small axoned ocellar neurone
- TTX :
-
tetrodotoxin
- 4-AP :
-
4-aminopyridine
References
Adams PR (1982) Voltage dependent conductances of vertebrate neurones. TINS 5:116–119
Adams PR, Halliwell JV (1982) A hyperpolarization induced inward current in hippocampal pyramidal cells. J Physiol 324:62–63P
Adams DJ, Smith SJ, Thompson SH (1980) Ionic currents in molluscan soma. Annu Rev Neurosci 3:141–167
Ammermüller J (1986) Passive cable properties of locust ocellar L-neurons. J Comp Physiol A 158:339–344
Ammermüller J, Weiler R (1985) S-neurons and not L-neurons are the source of GABAergic action in the ocellar retina. J Comp Physiol A 157:779–788
Bader CR, Bertrand D, Dupin E (1985) Voltage-dependent potassium currents in developing neurones from quail mesencephalic neural crest. J Physiol 366:129–151
Bezanilla F, Vergara J, Taylor RE (1982) Voltage clamping of excitable membranes. In: Ehrenstein G, Lecar H (eds) Biophysics (Methods of experimental physics, vol 20). Academic Press, New York, pp 445–511
Chang DC, Liu J (1985) A comparative study of the effects of tetrodotoxin and the removal of external Na on the resting potential: Evidence of separate pathways for the resting and excitable Na currents in squid axon. Cell Mol Neurobiol 5:311–320
Chappell RL, Dowling JE (1972) Neural organization of the median ocellus of the dragonfly. I. Intracellular electrical activity. J Gen Physiol 60:121–147
Connor JA, Stevens CF (1971) Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol 213:21–30
Galvan M, Sedlmeir C (1984) Outward currents in voltage-clamped rat sympathetic neurones. J Physiol 356:115–133
Goodman CS (1976) Anatomy of the ocellar interneurones of acridid grasshoppers. I. The large interneurones. Cell Tissue Res 175:183–202
Goodman LJ (1981) Organization and physiology of the insect dorsal ocellar system. In: Autrum H (ed) Vision in invertebrates (Handbook of sensory physiology, vol VII/6C). Springer, Berlin Heidelberg New York, pp 201–286
Goodman LJ, Mobbs PG, Guy RG (1976) Information processing along the course of a visual interneuron. Experientia 33:748–750
Gustafsson B, Galvan M, Grafe P, Wigström H (1982) A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature 299:252–254
Guy RG, Goodman LJ, Mobbs PG (1979) Visual interneurons in the bee brain: Synaptic organization and transmission by graded potentials. J Comp Physiol 134:263–264
Hausen K (1981) Monocular and binocular computation of motion in the lobula plate of the fly. Verh Dtsch Zool Ges 74:49–70
Hengstenberg R (1977) Spike responses of ‘non-spiking’ visual interneurons. Nature 270:338–340
Hodgkin AL, Huxley AF (1952) Currents carried by sodium and potassium ions through the membrane of the giant axon ofLoligo. J Physiol 116:449–472
Hodgkin AL, Huxley AF, Katz B (1952) Measurements of current-voltage relations in the membrane of the giant axons ofLoligo. J Physiol 116:424–448
Hume JR, Giles W (1983) Ionic currents in single isolated bullfrog atrial cells. J Gen Physiol 81:153–194
Jährvilehto M, Zettler F (1971) Localized intracellular potentials from pre- and postsynaptic components in the external plexiform layer of an insect retina. Z Vergl Physiol 75:422–446
Johnston D, Hablitz JJ, Wilson WA (1980) Voltage clamp discloses slow inward current in hippocampal burst-firing neurones. Nature 286:391–393
Kaneko A (1970) Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol 207:623–633
Kaneko A, Tachibana M (1985) A voltage-clamp analysis of membrane currents in solitary bipolar cells dissociated fromCarassius auratus. J Physiol 358:131–152
Klingman A, Chappell RL (1978) Feedback synaptic interaction in the dragonfly ocellar retina. J Gen Physiol 71:157–175
Kondo H (1978) Efferent system of the lateral ocellus in the dragonfly: Its relationship with the ocellar afferent units, the compound eyes and the wing sensory system. J Comp Physiol 125:341–349
Lasater EM (1986) Membrane properties of isolated cultured perch horizontal cells. Invest Ophthal Visual Sci 27 [Suppl]: 130
Lowe DA, Bush BMH, Ripley SH (1978) Pharmacological evidence for ‘fast’ sodium channels in nonspiking neurones. Nature 274:289–290
Milde JJ (1981) Graded potentials and action potentials in the large ocellar interneurons of the bee. J Comp Physiol 143:427–434
Milde JJ (1984) Ocellar interneurons in the honeybee. Structure and signals of L-neurons. J Comp Physiol A 154:683–693
Milde JJ, Homberg U (1984) Ocellar interneurons in the honeybee. Characteristics of spiking L-neurons. J Comp Physiol A 155:151–160
Mirolli M (1981) Fast inward and outward current channels in a non-spiking neurone. Nature 292:251–253
Mirolli M (1983) Inward and outward currents in isolated dendrites of crustacean coxal receptors. Cell Mol Neurobiol 3:355–370
Mizunami M, Yamashita S, Tateda H (1982) Intracellular stainings of the large ocellar second order neurons in the cockroach. J Comp Physiol 149:215–219
Narahashi T (1974) Chemicals as tools in the study of excitable membranes. Physiol Rev 54:813–889
Neher E (1971) Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol 58:36–53
Oertel D, Stuart AE (1981) Transformation of signals by interneurones in the barnacle's visual pathway. J Physiol 311:127–146
Patterson JA, Goodman LJ (1974) Intracellular responses of receptor cells and second-order cells in the ocelli of the desert locustSchistocerca gregaria J Comp Physiol 95:237–250
Patterson JA, Chappell RL (1980) Intracellular responses of Procion filled cells and whole nerve cobalt impregnation in the dragonfly median ocellus. J Comp Physiol 139:25–39
Pearson KG, Fourtner CR (1975) Nonspiking interneurons in walking system of the cockroach. J Neurophysiol 38:33–52
Pelhate M, Sattelle DB (1982) Pharmacological properties of insect axons: a review. J Insect Physiol 28:889–903
Pichon Y (1974) Axonal conduction in insects. In: Treherne JE (ed) Insect neurobiology. North Holland Publishing Comp, Amsterdam, pp 73–117
Pichon Y, Ashcroft M (1985) Nerve and muscle: electrical activity. In: Kerkut GA, Gilbert LL (eds) Nervous system: structure and motor function (Comprehensive insect physiology, biochemistry and pharmacology, vol 5). Pergamon Press, Oxford New York Toronto Sidney Paris Frankfurt, pp 85–113
Rall W (1977) Core conductor theory and cable properties of neurons. In: Kandel ER (ed) The nervous system: cellular biology of neurons (Handbook of physiology, sect 1, vol 1, part 1). Am Physiol Soc, Bethesda, pp 39–98
Salkoff LB, Wyman RJ (1983) Ion currents inDrosophila flight muscles. J Physiol 337:687–709
Schwarz W, Passow H (1983) Ca2+-activated K+ channels in erythrocytes and excitable cells. Annu Rev Physiol 45:359–374
Segal M, Barker JL (1984) Rat hippocampal neurons in culture: potassium conductances. J Neurophysiol 51:1409–1433
Segal M, Rogawski MA, Barker JL (1984) A transient potassium conductance regulates the excitability of cultured hippocampal and spinal neurons. J Neurosci 4:604–609
Shingai R, Christensen BN (1983) Sodium and calcium currents measured in isolated catfish horizontal cells under voltage clamp. Neuroscience 10:893–897
Siegler MVS (1985) Nonspiking interneurons and motor control in insects. Adv Insect Physiol 18:249–304
Simmons PJ (1981) Synaptic transmission between second- and third-order neurones of a locust ocellus. J Comp Physiol 145:265–276
Simmons PJ (1982a) Transmission mediated with and without spikes at connexions between large second-order neurones of locust ocelli. J Comp Physiol 147:401–414
Simmons PJ (1982b) The operation of connexions between photoreceptors and large second-order neurones in dragonfly ocelli. J Comp Physiol 149:189–398
Simmons PJ (1985) Postsynaptic potentials of limited duration in visual neurones of a locust. J Exp Biol 117:193–213
Tachibana M (1983b) Ionic currents of solitary horizontal cells isolated from goldfish retina. J Physiol 345:329–351
Tachibana M (1983b) Solitary horizontal cells in culture. I. Their electrical properties. Vision Res 23:1209–1216
Thompson S (1982) Aminopyridine block of transient potassium current. J Gen Physiol 80:1–18
Treherne JE, Schofield PK (1981) Mechanisms of ionic homeostasis in the central nervous system of an insect. J Exp Biol 95:61–73
Werblin FS, Dowling JE (1969) Organization of the retina of the mudpuppy,Necturus maculosus. II. Intracellular recording. J Neurophysiol 32:339–355
Wilson M (1978a) The functional organization of locust ocelli. J Comp Physiol 124:297–316
Wilson M (1978b) Generation of graded potential signals in the second order cells of locust ocellus. J Comp Physiol 124:317–331
Wilson M (1978c) The origin and properties of discrete hyperpolarizing potentials in the second order cells of locust ocellus. J Comp Physiol 128:347–358
Wu C-F, Haugland FN (1985) Voltage clamp analysis of membrane currents in larval muscle fibers ofDrosophila: Alteration of potassium currents in shaker mutants. J Neurosci 5:2626–2640
Zettler F, Weiler R (1976) Neuronal processing in the first optic neuropil of the compound eye of the fly. In: Zettler F, Weiler R (eds) Neural principles in vision. Springer, Berlin Heidelberg New York, pp 227–237
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ammermüller, J., Zettler, F. Time- and voltage-dependent currents in locust ocellar L-neurones. J. Comp. Physiol. 159, 363–376 (1986). https://doi.org/10.1007/BF00603982
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00603982