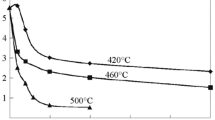
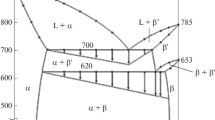
Abstract
Investigations were carried out on a model system Ag-Zn-S and consisted of determinations of kinetic data, and distribution of the chemical potential of silver in the scale, as well as the amount of inward diffusion of sulfur in the formation of two-layer scales on binary alloys. The chemical potential of silver was measured with AgI as a solid electrolyte and the diffusion of sulfur with the aid of the radioactive isotope35S. The inner layer consisted of pellets of Ag2S+ZnS mixtures having different degrees of dispersion and of mixtures of Ag2S and powdered foam glass. The investigations were carried out on the following systems: Ag-Ag2S-S; Ag-Ag2S+ ZnS-Ag2S-S; Ag-Ag2S+ glass-Ag2S-S; (Ag-Zn)-Ag2S-S. It was established that the ability of dispersed ZnS to decrease the rate can be explained on the basis of a decreasing cross section for the outward diffusion of silver ions in the inner layer. This phase makes the plastic flow of the scale toward the core very difficult. As a result of this, the secondary dissociation processes destroy the compactness of the scale over the whole cross section and favor the inward diffusion of sulfur. The chemical potential varies linearly across each layer.
Similar content being viewed by others
References
C. Wagner,Z. Physik. Chem. (Leipzig) 21, 25 (1933).
H. Rickert,Z. Physik. Chem. (Frankfurt) 23, 355 (1960).
S. Mrowec,Roczniki Chem. 37, 207 (1963).
A. Brückman,Corrosion Sci. 7, 51 (1967).
S. Mrowec.Corrosion Sci. 7, 563 (1967).
K. Fueki and J. B. Wagner,J. Electrochem. Soc. 112, 384 (1965).
A. Brückman, S. Mrowec, and T. Werber,Fiz. Metal, i Metalloved. 15, 362 (1963);20, 702 (1965).
S. Mrowec, T. Wanalec, T. Werber,Corrosion Sci. 6, 287 (1966).
R. Meussner and O. Birchenall,Corrosion 13, 677 (1955).
L. Czerski, S. Mrowec, and T. Werber,J. Electrochem. Soc. 109, 273 (1962).
H. Engell and F. Weber,Acta Met. 5, 695 (1957).
S. Mrowec and T. Werber,Corrosion Sci. 5, 11 (1965).
A. Brückman and J. Romanski,Corrosion Sci. 5, 185 (1965).
S. Mrowec and T. Werber,Werkstoffe Korrosion 18, 116 (1967);19, 944 (1968).
A. Brückman, S. Mrowec, and T. Werber,Z. Physik. Chem. (Leipzig) 231, 375 (1966).
S. Mrowec and H. Rickert,Z. Physik. Chem. (Frankfurt) 28, 422 (1961).
R. L. Allen and W. J. Moore,J. Phys. Chem. 63, 223 (1959).
C. Wagner, Diffusion and high temperature oxidation of metals,Atom Movements, John Holloman, ed. (American Society for Metals, Cleveland, Ohio, 1951), p. 153.
I. Bartkowicz, S. Mrowec, and T. Werber,Bull. Acad. Polon. Sci. Ser. Sci. Chim. 15, 537 1967.
L. Czerski, S. Mrowec, K. Wallish, and T. Werber,Arch. Hutnictwa 3, 49 1958.
J. Mikulski, S. Mrowec, and T. Werber,Bull. Acad. Polon. Sci. Ser. Sci. Chim. 7, 737 (1959).
J. Gilewicz-Wolter,Zeszyty Nauk. Akad. Gorniczo-Hutniczej Krakowie Ceram. (in press).
S. Mrowec and H. Rickert,Z. Physik. Chem. (Frankfurt) 32, 212 (1962).
I. Bartkowicz and S. Mrowec,Bull. Acad. Polon. Sci. Ser. Sci. Chim. (in press).
K. Kiukkola and C. Wagner,J. Electrochem. Soc. 104, 379 (1957).
S. Mrowec, K. Wallish, and T. Werber,Arch. Hutnictwa 10, 295 (1965).
S. Mrowec,Arch. Hutnictwa 6, 327 (1961).
S. Mrowec and T. Werber,Chem. Stosowana 1, 65 (1965).
J. Mikulski, S. Mrowec, and T. Werber,Bull. Acad. Polon. Sci. Ser. Sci. Math. Astron. Phys. 8, 179 (1960).
S. Mrowec, T. Werber, and J. Podhorodecki,Corrosion Sci. 8, 815 (1968).
S. Mrowec, T. Werber, and M. Zastawnik,Corrision Sci. 6, 47 (1966).
S. Mrowec, S. Tochowicz, T. Werber, and J. Podhorodecki,Corrosion Sci. 7, 697 (1967).
L. Czerski, S. Mrowec, and T. Werber,Arch. Hutnictwa 4, 245 (1959).
J. Sartell, S. Bendel, T. Johnston, and C. Li,Trans. Am. Soc. Metals 50, 1047 (1958).
S. Mrowec and T. Werber,Acta Met. 7, 696 (1959).
S. Mrowec,Arch. Hutnictwa 6, 61 (1961).
J. Mikulski, S. Mrowec, I. Stronski, and T. Werber,Z. Physik. Chem. (Frankfurt)22, 20 (1959).
H. Engell,Acta Met. 6, 439 (1958).
F. Pettit,J. Electrochem. Soc. 113, 1249 (1966).
S. Mrowec,Bull Acad. Polon. Sci. Ser. Sci. Chim. 15, 373 (1967).
S. Mrowec,Bull. Acad. Polon. Sci. Ser. Sci. Chim. 15, 527 (1967).
J. Bardeen, W. H. Brattain, and W. Shockley,J. Chem. Phys. 14, 714 (1946).
P. Rahlfs,Z. Physik. Chem. (Leipzig) B31 157 (1936).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brückman, A., Gilewicz-Welter, J. & Mrowec, S. Application of Wagner's pellet method in investigating the mechanism of formation of heterophasic two-layer scales on alloys. Oxid Met 1, 241–265 (1969). https://doi.org/10.1007/BF00603518
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00603518