Abstract
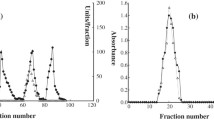
The diaphorase activities of the proteins of different varieties of two species of cotton plant —Gossypium hirsutum L. andG. barbadense L. — have been studied. It has been shown that the proteins of the seeds of the cotton plantG. barbadense possess a low diaphorase activity in comparison with the proteins of the seeds of aG. hirsutum plant. On an electrophoretogram of the proteins, diaphorase activity was localized in two zones, with Rf 0.45 and 0.70. The diaphorase with Rf 0.45 has been isolated by electrophoresis in polyacrylamide gel (PAAG) and some of its properties have been studied. The diaphorase isolated oxidizes NADH and NADPH in the presence of various artificial electron Acceptors, and has two pH optima (at 7.20 and 8.70) and is characterized by relative thermal stability (at 80°C). In the case of the total extract, brief boiling does not lead to inactivation of the enzyme, which shows the presence in cotton seeds of a factor stabilizing this diaphorase. The molecular weight of the proteins isolated, according to gel filtration on Sephadex G-150, is 59,000, and from the results of SDS-PAAG electrophoresis it is 13,600, which shows a tetrameric structure of the enzyme.
Similar content being viewed by others
Literature cited
Sh. Yunuskhanov and A. P. Ibragimov, Dokl. Akad. Nauk UzbSSR, No. 7, 50 (1983).
Sh. Yunuskhanov and B. D. Dzhalilov, Khim. Prir. Soedin., 488 (1986).
Sh. Yunuskhanov and A. P. Ibragimov, Genetika,20, No. 6, 989 (1984).
A. S. Purvis, C. R. Tischler, and E. A. Funkhouser, Physiol. Plant, 48, No. 3, 389 (1980).
L. Ernster, Methods Enzymol., R. Estabroolt and M. E. Pullman, (eds.),10, 309 (1967).
C. Martius, in: The Enzymes, P. D. Boyer, L. Lordy, and K. Myrback (eds.), Academic Press, New York, Vol. 7, Part A (1963).
N. P. L'vov, Sh. S. Burikhanov, and V. L. Kretovich, Prikl. Biokhim. Mikrobiol.,16, No. 6, 805–818 (1980).
J. H. Sherrard and M. J. Dalling, Plant Physiol.,63, 346 (1970).
M. G. Redinbaugh and W. H. Campbell, Plant Physiol,68, 115 (1981).
P. A. Kolesnikov, S. V. Zoré, K. V. Pshenova, E. I. Petrochenko, N. K. Pletnikova, L. E. Makovkina, and A. A. Mutuskin, Fiziol. Rast (Moscow),20, No. 1, 170 (1973).
T. Borner, Biochem. Physiol. Pflanzen,136, No. 7, 667 (1981).
Sh. Yunuskhanov, in: Methods for the Physiological-Biochemical Investigation of the Cotton Plant [in Russian], Tashkent (1973), p. 28.
O. H. Lowry, N. J. Rosebrough, A. L. Farr, and R. J. Randall, J. Biol. Chem.,193, No. 1, 265 (1951).
B. J. Davis, Ann. New York Acad. Sci.,121, No. 2, 404 (1964).
U. K. Laemmli and M. Favre, J. Mol. Biol.,80, No. 2, 757 (1973).
R. Schneeman and D. W. Krogman, J. Biol. Chem.,250, 2965 (1975).
Additional information
Institute of Experimental Plant Biology, Academy of Sciences of the Uzbek SSR, Tashkent. Translated from Khimiya Prirodnykh Soedinenii, No. 3, pp. 416–421, May–June, 1987.
Rights and permissions
About this article
Cite this article
Yunuskhanov, S. Isolation of a protein with diaphorase activity from cotton seeds and a study of some of its properties. Chem Nat Compd 23, 344–348 (1987). https://doi.org/10.1007/BF00600837
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00600837