Abstract
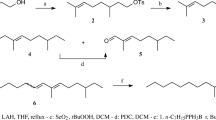
A synthesis of hexadec-9Z-enal — a component of the sex pheromone of the cotton boll-wormHeliothis armigera (Hübner) — based on cyclooctene (I) is proposed. Through a solution of 22 g of (I), 250 ml of cyclohexane, and 40 ml of MeOH is passed (at 5°C) 0.2 M O3/O2, the solution is decanted off, and the precipitated ozonide is dissolved in 200 ml of MeOH and is reduced with 19 g of NaBH4 (40°C) with the isolation, after the usual working up, of 23.4 g of octane-1,8-diol (II). From 0.5 mole of (II) and 0.6 mole of 45% HBr 8-bromooctan-1-ol (III) is obtained and this is converted into 1-(2-THPL)oxy)-8-bromooctane (IV). The condensation of (IV) with oct-1-yne (Ar, LiNH2, HMPTA, 10°C, 1 h, and then 55°C, 10 h) leads to 1-(2-THPL-oxy)hexadec-9-yne (V) the hydrolysis of which (MeOH, H2O, p-TsOH, 20°C for 20 h) yields hexadec-9-yn-ol (VI). The reduction of (VI) (Et2O, iso-BuMgBr, Cp2TiCl2, 0°C, 15 min, then 20°C, 1 h) yieldshexadec-9Z-en-l-ol (VII). The oxidation of (VII) (PyHCrO +3 Cl−, CH2Cl2, 20°C, 2 h) gives hexadex-9Z-enal (VIII). Characteristics of the compounds (yield (%), n 20D (25): (II) − 80, mp 61–62°C; (III) − 75, 1.4807; (IV) − 99, —; (V) − 52, 1,4650; (VI) − 85, 1.4657; (VII) − 99, 1.4650; (VIII) − 98, 1.4600. Characteristics of the IR and PMR spectra of compounds (V–VII) are given.
Similar content being viewed by others
Literature cited
V. V. Buleza, E. R. Myttus, A. S. Kovaleva, M. Z. Kogan, V. D. Kravchenko, and S. G. Apasov, Dokl. Akad. Nauk SSSR,272, 244 (1983).
C. A. Hendrick, Tetrahedron,33, 1845 (1977).
V. P. Konyukhov, B. G. Kovalev, V. A. Minyailo, V. V. Stan, and Yu. F. Oprunenko, Khemoretseptsiya Nasekomykh, No. 3, 37 (1978).
L. Liu, G. Lin, X. Wang, and G. Guo, Huaxuc Xuebao,43, No. 4, 400 (1985). Chem. Abstr.,103, 215029 (1985).
R. Rossi, A. Carpits, and M. L. Gaudenzi, Synthesis, No. 5, 359 (1981).
O. L. Chapman, K. C. Mattes, R. S. Sheridan, and A. N. Kishaba, J. Am. Chem. Soc.,100, 4878 (1978).
Additional information
Institute of Chemistry, Bashkir Branch, Academy of Sciences of the USSR, Ufa. Translated from Khimiya Prirodnykh Soedinenii, No. 2, pp. 286–289, March–April, 1987.
Rights and permissions
About this article
Cite this article
Odinolov, V.N., Dzhemilev, U.M., Ishmuratov, G.Y. et al. Insect pheromones and their analogues. XVI. Practical synthesis of hexadec-9Z-enal — A component of the sex pheromone of the cotton bollwormHeliothis armigera . Chem Nat Compd 23, 242–244 (1987). https://doi.org/10.1007/BF00598768
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00598768