Abstract
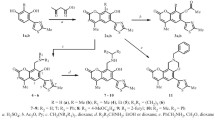
On performing the modification of furanoeremophilan-14β,6α-olide at the most reactive fragments of the molecule, the lactone and furan rings, it was established that in an alkaline medium furanoeremophilan-14β,6α-olide isomerizes into the 14α,6α-olide; the oxidation of furanoeremophilan-14β,6α-olide to a dilactone in the presence of Rose Bengal takes place with high yield under mild conditions — atmospheric oxygen, without heating and irradiation; on the interaction of furanoeremophilan-14β,6α-olide with selenium dioxide in glacial acetic acid a selenium-containing dimer is formed.
Similar content being viewed by others
Literature cited
H. Ishii, T. Tozyo, and H. Minato, Tetrahedron,21, 2605 (1965).
Y. Tanahshi, W. Masatke, and T. Takahashi, Bull. Soc. Chim. France,3, 1047 (1968).
M. Tada, Y. Moriyama, Y. Tanahshi, and T. Takahashi, Tetrahedron Lett., No. 43, 4007 (1971).
H. Nagano and T. Takahashi, Bull. Chem. Soc. Jpn.,45, 1935 (1972).
T. Sato, Y. Moriyama, H. Nagano, Y. Tanahashi, and Y. Takahashi, Bull. Chem. Soc. Jpn.,48, 112 (1975).
F. Bohlmann, D. Ehlers, Ch. Zdero, and N. Grenz, Chem. Ber.110, 2640 (1977).
H. Nagano and T. Takahashi, Bull. Chem. Soc. Jpn.,51, No. 11, 3335 (1978).
F. Bohlmann and M. Grenz, Phytochemistry,18, 491 (1979).
Y. Ishizaki, Y. Tanahashi, T. Tsuyuki, T. Takahashi, and K. Tori, Bull. Chem. Soc. Jpn.,52, No. 4, 1182 (1979).
Y. Moriyama and T. Takahashi, Bull. Chem. Soc. Jpn.,49, No. 11, 3196 (1976).
L. P. Nikonova and G. K. Nikonov, Khim. Prir. Soedin., 742 (1976).
K. A. Abdykalikova and G. K. Nikonov, Vestnik Akad. Nauk KazSSR,9, 51 (1987).
L. M. Kataeva, I. V. Anonimova, L. K. Yuldasheva, and E. G. Kataev, Zh. Obshch. Khim.,32, (XCIV), 3965 (1962).
V. V. Tkachev, G. K. Nikonov, L. O. Atovmyan, A. Ya. Kobzar', and T. V. Zinchenko, Khim. Prir. Soedin., 811 (1987).
J. H. Beynon, Mass Spectrometry and Its Applications to Organic Chemistry, Elsevier, Amsterdam (1960) [Russian translation, Mir, Moscow (1964), p. 573].
G. Brauer, A Handbook of Preparative Inorganic Chemistry [Russian translation from the 1st German edition], IL, Moscow (1956), p. 217.
Additional information
Institute of Chemical Sciences of the Kazakh SSR Academy of Sciences, Alma-Ata. Translated from Khimiya Prirodnykh Soedinenii, No. 2, pp. 189–193, March–April, 1989.
Rights and permissions
About this article
Cite this article
Abdykalikova, K.A., Nikonov, G.K. & Zamkova, V.V. Chemical modification of furanoeremophilan-14β,6α-olide. Chem Nat Compd 25, 160–164 (1989). https://doi.org/10.1007/BF00598402
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00598402