Abstract
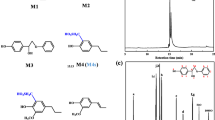
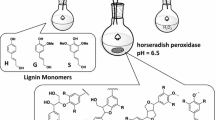
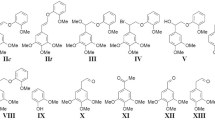
Using α-guaiacylpropanone as an example, it has been shown that, in the process of peroxidase oxidation of carbonyl-containing structures, biphenyl compounds having C-C bonds in the 5-5′ positions are formed. On the basis of the results of physicochemical methods of analysis, the formation of biphenyl spatially hindered structures with screened radical centers is suggested.
Similar content being viewed by others
Literature cited
V. A. Strel'skii and É. I. Chupka, Khim. Prir. Soedin., No. 1, 115 (1988).
J. A. Barltrop and J. D. Koyle, Excited States in Organic Chemistry, Wiley-Interscience, New York (1977).
E. S. Caldwell and C. Steelink, Biochim. Biophys. Acta,184, 43 (1969).
O. N. Grushnikov and V. V. Elkin, Advances in and Problems of Lignin Chemistry [in Russian], Moscow (1973).
Additional information
Siberian Scientific-Research Institute of Cellulose and Cardboard, Bratsk. Translated from Khimiya Prirodnykh Soedinenii, No. 1, pp. 111–115, January–February, 1988.
Rights and permissions
About this article
Cite this article
Strel'skii, V.A., Chupka, E.I. Enzymatic oxidation of lignin and compounds modeling it. V. Products of peroxidase oxidation of α-guaiacylpropanone. Chem Nat Compd 24, 99–101 (1988). https://doi.org/10.1007/BF00597585
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00597585