Summary
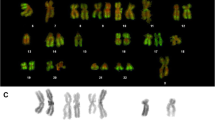
Antibodies against Z-DNA react with fixed metaphase chromosomes of man and other mammals. Indirect immunofluorescence staining shows that chromosomal segments corresponding to R- and T-bands preferentially fix Z-DNA antibodies. In this work Z-DNA antibodies were used as a probe for DNA conformation in euchromatin of fixed human chromosomes whose condensation or staining were modified by actinomycin D (AMD) and by 5-bromodeoxyuridine (BrdU). Treatments with AMD and BrdU were performed to induce a G-banding by modification of chromosomal segments corresponding to R- and T-bands. Long BrdU treatments were used to induce asymmetrical and partially undercondensed chromosomes by substitution of thymidine in one or both DNA strand. Our results show a clear difference of Z-DNA antibodies reactivity after AMD or BrdU treatment. The G-banding obtained after AMD treatment is not reversed by Z-DNA antibodies staining since these antibodies bind very weakly to the undercondensed R-bands. On the other hand, the G-banding obtained by BrdU is completely reversed giving typical R-banding, as on untreated chromosomes. For asymmetrical chromosomes an R-, T-banding pattern is always observed but there is a decrease of the fluorescence intensity proportional to the degree of BrdU incorporation. We conclude that AMD treatment greatly disturbs Z-DNA antibodies binding suggesting a change in DNA conformation, whereas BrdU treatments do not suppress but only weaken the specific binding of Z-DNA antibodies on R- and T-bands. The direct involvement of thymidine substitution in DNA sequences recognized by Z-DNA antibodies is discussed.
Similar content being viewed by others
References
Arndt-Jovin DJ, Robert-Nicoud M, Zarling D, Greider C, Weimer E, Jovin MT (1983) Left-handed Z-DNA in bands of acid-fixed polytene chromosomes. Proc Natl Acad Sci USA 80:4344–4348
Benyajati C, Worcel A (1976) Isolation, characterization and structure of the folded interphase genome ofDrosophila melanogaster. Cell 9:393–407
Buys CH, Osinga J (1980) The mechanism of differential sister chromatid fluorescence as studied with the G-C specific DNA ligant mithramycin. Exp Cell Res 125:105–109
Couturier J, Morichon-Delvallez N, Dutrillaux B (1985) Deletion of band 13q21 is compatible with normal phenotype. Hum Genet 70: 87–91
Dutrillaux B, Viegas-Péquinot E (1981) High resolution R- and G-banding on the same preparation. Hum Genet 57:93–95
Dutrillaux B, Fosse AM, Prieur M, Lejeune J (1974) Analyse des échanges de chromatides dans les cellules somatiques humaines. Traitement au BrdU (5-bromodéoxyuridine) et fluorescence bicolore par l'acridine orange. Chromosoma 48:327–340
Dutrillaux B, Aurias A, Fosse AM (1976a) Différentiation des mécanismes induisants la segmentation et l'asymétrie des chromatides, après traintements par le 5-bromodéoxyuridine. Exp Cell Res 97:313–321
Dutrillaux B, Couturier J, Richer CL, Viegas-Péquignot E (1976b) Sequence of DNA replication in 277 R- and Q-bands of human chromosomes using a BrdU treatment. Chromosoma 58:51–61
Goldman MA, Holmquist GP, Gray MC, Caston LA, Nag A (1984) Replication timing of genes and middle repetitive sequences. Science 224:686–691
Grezeschik KH, Kim MA, Johannsmann R (1975) Late replication bands of human chromosomes demonstrated by fluorochrome and Giemsa staining. Humangenetik 29:41–59
Hamada H, Kakamaga T (1982) Potential Z-DNA forming sequences are highly dispersed in human genome. Nature 298:391–398
Hamada H, Petrino MG, Kakamaga T, Seidman M, Stollar BD (1984) Characterization of genomic poly (dT-dG) poly (dC-dA) sequences: structure, organization and conformation. Mol Cell Biol 4:2610–1621
Haniford DB, Pulleybland D (1983) Facile transition of poly (d(TG) d(CA)) into a left-handed helix in physiological conditions. Nature 320:632–634
Hill RJ, Stollar BD (1983) Dependence of Z-DNA antibody binding to polytene chromosomes on acid fixation and DNA torsional strain. Nature 305:338–340
Hill RJ, Watt F, Stollar D (1984) Z-DNA immunoreactivity ofDrosophila polytene chromosomes. Effects of the fixatives 45% acetic acid and 95% ethanol and of DNase 1 nickling. Exp Cell Res 153: 469–482
Hsu TC, Pathak S, Shafer DA (1973) Induction of chromosome crossbanding by trealing cells with chemical agents before fixation. Exp Cell Res 79:484–487
Human Gene Mapping 7, 1983 (1984) Cytogenet Cell Genet 37:1–666
Igo-Kemenes T, Zachan JJ (1978) Domains in chromatin structure. Cold Spring Harbor Symp Quant Biol 42:109–118
Javamerian K, Liu LF, Wang JC (1978) Non-histone proteins HMG1 and HMG2 change the DNA helical structure. Science 199:1345–1346
Klysik J, Stirdivant SM, Larson JE, Hart PA, Wells RD (1981) Lefthanded DNA in restriction fragments and a recombinant plasmid. Nature 290:672–677
Lang MC, Malfoy B, Freund AM, Daune M, Leng M (1982) Visualization of 2 sequences in form V of pBR 322 by immunoelectron microscopy. EMBO J 1:1149–1153
Latt SA (1973) Microfluorometric detection of deoxyribonucleic acid replication in human metaphase chromosomes. Proc Natl Acad Sci USA 70:3395–3399
Lemeunier F, Derbin C, Malfoy B, Leng M, Taillandier E (1982) Identification of left-handed Z-DNA by indirect immunofluorescence in polytene chromosomes ofChironomus thummi thummi. Exp Cell Res 141:508–513
Leng M (1985) Left handed Z-DNA. Biochim Biophys Acta 825:339–344
Lipps HJ, Nordheim A, Lafer EM, Ammermann D, Stollar BD, Rich A (1983) Antibodies against Z-DNA react with the macronucleus but not the micronucleus of the hypotrichous ciliateStylonychia mytilus. Cell 32:435–441
Malfoy B, Rousseau N, Leng M (1982) Interaction between antibodies to Z form deoxyribonucleic acid and double-stranded polynucleotide. Biochemistry 21:5463–5467
Malfoy M, Rousseau N, Viegas-Péquignot E, Dutrillaux B, Leng M, (1986) Nucleotide sequence of an heterochromatic segment recognized by the antibodies to Z-DNA in fixed metaphase chromosomes. Nucleic Acids Res 14:3197–3214
Morgenegg G, Celio MR, Malfoy B, Leng M, Kuenzle CC (1983) Z-DNA immunoreactivity in rat tissues. Nature 303:540–543
Nordheim A, Rich A (1983) Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature 303:674–679
Nordheim AM, Pardue ML, Lafer EM, Möller A, Rich A (1981) Antibodies to left handed Z-DNA bind to interband regions ofDrosophila polytene chromosomes. Nature 294:417–422
Nordheim A, Lafer EM, Peck LJ, Wang JC, Stollar BD, Rich A (1982) Negatively supercoiled plasmids contein left-handed Z-DNA segments as detected by specific antibody binding. Cell 31: 309–318
Nordheim A, Peck LJ, Lafer EM, Stollar BD, Wang JC, Rich A (1983) Supercoiling and left handed Z-DNA. Cold Spring Harbor Symp Quant Biol 47:93–100
Oudet P, Gross-Bellard M, Chambon P (1975) Folding of the DNA double helix in chromatin. Cell 4:281–300
Patel DJ, Kozlowski SA, Rice JA, Broka C, Itakura K (1981) Mutual interaction between adjacent dG.dC actinomycin binding sites and dA.dT metropsin binding sites on the self-complementary (d C-G-C-G-A-A-T-T-C-G-C-G) duplex in solution. Proc Natl Acad Sci USA 78:7281–7284
Peck LJ, Nordheim A, Rich A, Wang JC (1982) Flipping of cloned d(pCpG) d(pCpG)n DNA sequences from right to left-handed helical structure by salt, Co(III) or negative supercoiling. Proc Natl Acad Sci USA 79:4560–4564
Perry P, Wolff S (1974) New Giemsa method for the differential staining of sister chromatids. Nature 251:156–158
Rich A, Nordheim A, Wang AHJ (1984) The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem 53:791–846
Robert-Nicoud M, Arndt-Jovin DJ, Zarling DA, Jovin TM (1984) Immunological detection of left handed Z-DNA in isolated polytene chromosomes. Effects of ionic strength, pH, temperature and topological stress. EMBO J 3:721–731
Shafer DA (1973) Banding human chromosomes in culture with actinomycin. Lancet I:828
Singleton CK, Klysik J, Stirdivant SM, Wells RP (1982) Left-handed Z-DNA is induced by supercoiling in physiological ionic conditions. Nature 299:312–316
Staiano-Coico L, Stollar BD, Darzynkiewicz Z, Dutkowski R, Weksler ME (1985) Binding of anti-Z-DNA antibodies in quiescent and activated lymphocytes: relationship to cell cycle progression and chromatin changes. Mol Cell Biol 5:3270–3273
Van de Sande JH, Jovin TM (1982) Z-DNA, the left-handed helical form of poly (d(G-C)) in Mg Cl2-ethanol, is biologically active. EMBO J 1:115–120
Viegas-Péquignot E, Dutrillaux B (1976) Modification spécifique des bandes R et Q par le 5-bromodéoxyuridine et l'actinomycine D. Exp Cell Res 98:338–348
Viegas-Péquignot E, Derbin C, Lemeunier F, Taillandier E (1982) Identification of left handed Z-DNA by indirect immunomethods in metaphasic chromosomes of a mammal,Gerbillus nigeriae (Gerbillidae, Rodentia). Ann Génét (Paris) 25:218–222
Viegas-Péquignot E, Derbin C, Malfoy B, Taillandier E, Dutrillaux B, (1983) Z-DNA immunoreactivity in fixed metaphase chromosomes of primates. Proc. Natl Acad Sci USA 80:5890–5894
Viegas-Péquignot E, Malfoy B, Leng M, Dutrillaux B, Tchen P (1986) In situ hybridization of an acetylaminofluorene-modified probe recognized by Z-DNA antibodies in vitro. Cytogenet Cell Genet 42:105–109
Weisbrod S (1982) Active chromatin. Nature 297:289–295
Zakharov AF, Egolina NA (1972) Differential spiralisation along mammalian mitotic chromosomes. Chromosoma 38:341–365
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Viegas-Pequignot, E., Malfoy, B., Sabatier, L. et al. Different reactivity of Z-DNA antibodies with human chromosomes modified by actinomycin D and 5-bromodeoxyuridine. Hum Genet 75, 114–119 (1987). https://doi.org/10.1007/BF00591070
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00591070