Abstract
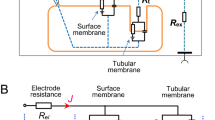
The length dependence of the voltage divider ratio (VDR) was investigated in a double cable model of tubular epithelia with point source current injection into the tubular lumen in order to find out, whether there is a region, in which the VDR — as in flat sheet epithelia — is an appropriate measure of the relative magnitude of the apical (r a) and basal (r b) cell membrane resistances. Irrespective of the choice of the cable parameters, we find that VDR, defined as luminal over cellular voltage deflection, overestimates the resistance ratio (r a+r b):r b near the origin, but underestimates it at distances (χ) greater than 1 luminal length constant (β). In the region χ<β there is a crossover point, where VDR is an accurate estimate of the resistance ratio. If the difference between VDR at the origin and at large distances (χ>β) is small, then VDR is a good estimate of the resistance ratio. This is also true, if VDR is constant between χ∼0.5 β and χ>β, (with the exception of some cases, in which the longitudinal resistance in the cell column is exceedingly high). If the latter conditions do not apply, we find that VDR, as measured at χ=β, underestimates the resistance ratio at worst only by 8.8%, provided the cable properties are such that the luminal voltage attenuation exhibits only one single exponential (with maximum tolerable amplitude deviation of 5% at the origin). Cable analysis measurements on rat proximal tubule indicate that VDR is constant in the range between χ∼0.5 β and χ>β. Hence (VDR)χ=β may be considered as a valid approximation of the ratio of cell membrane resistances in this epithelium.
Similar content being viewed by others
Abbreviations
- r a :
-
resistance per unit length of luminal membrane (Ω cm)
- r b :
-
resistance per unit length of basal membrane (Ω cm)
- r s :
-
resistance per unit length of shunt pathway (Ω cm)
- r i :
-
core resistance of luminal cable per unit length (Ω/cm)
- r c :
-
core resistance of cellular cable per unit length (Ω/cm)
- χ:
-
distance from current source (cm)
- V i :
-
the change in the lumen potential due to current injection (V)
- V c :
-
the change in the cellular potential due to current injection (V)
- I o :
-
injected current (A)
- λi :
-
length constant of the luminal cable if isolated from the cellular cable (cm)
- λc :
-
length constant of the cellular cable if isolated from the luminal cable (cm)
- α, β:
-
length constants actually observed in the double cable (cm)
- i a :
-
current flow through the apical resistance at χ (A/cm)
- i b :
-
current flow through the basal resistance at χ (A/cm)
- i s :
-
current flow through the shunt pathway at χ (A/cm)
- i c :
-
current flow along the cellular cable at χ (A)
- i i :
-
current flow along the luminal cable at χ (A)
References
Anagnostopoulos T, Teulon J, Edelman A (1980) Conductive properties of the proximal tubule inNecturus kidney. J Gen Physiol 75:553–587
Boulpaep EL, Sackin M (1980) Electrical analysis of intraepithelial barriers. In: Kleinzeller A, Bronner F, Boulpaep EL (eds) Current topics in membranes and transport, vol 13. Academic, New York, pp 169–197
Fatt P, Katz B (1953) The electrical properties of Crustacean muscle fibers. J Physiol 120:171–204
Frömter E (1972) The route of passive ion movement through the epithelium ofNecturus gallbladder. J Membrane Biol 8:259–301
Frömter E (1981) Electrophysiological analysis of rat renal sugar and amino acid transport. I. Basic phenomena. Pflügers Arch 393:179–189
Frömter E, Gebler B, Schopow K, Pockrandt-Hemstedt H (1974) Cation and anion permeability of rabbit submaxillary main duct. In: Thorn NA, Petersen OH (eds) Alfred Benzon Symp. VII Secretory mechanisms of exocrine glands. Munksgaard, Copenhagen, Acad Press, New York, pp 496–513
Frömter E, Müller CW, Wick I (1971) Permeability properties of the proximal tubular epithelium of the rat kidney studied with electrophysiological methods. In: Giebisch G (ed) Electrophysiology of epithelia. Schattauer, Stuttgart New York, pp 119–146
Greger R, Schlatter E (1983) Properties of the lumen membrane of the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflügers Arch 396:315–324.
Guggino WB, Windhager EE, Boulpaep EL, Giebisch G (1982) Cellular and paracellular resistances of theNecturus proximal tubule. J Membr Biol 67:143–154
Hoshi T, Kawahara K, Yokoyama R, Suenaga K (1981) Changes in membrane resistances of renal proximal tubule induced by cotransport of sodium and organic solute. Adv Physiol Sci (Akademiaí Kiado, Budapest) Vol 11:403–407
Koeppen BM, Biagi BA, Giebisch GH (1983) Intracellular microelectrode characterization of the rabbit cortical collecting duct. Am J Physiol 244:F35-F47
Kottra G, Frömter E (1984) Rapid determination of intraepithelial resistance barriers by alternating current spectroscopy. I. Experimental procedures. Pflügers Arch 402: 409–420
Kottra G, Frömter E (1984) Rapid determination of intraepithelial resistance barriers by alternating current spectroscopy. II. Test of model circuits and quantification of results. Pflügers Arch 402:421–432
Reuss L, Finn AL (1975) Electrical properties of the cellular transepithelial pathway inNecturus gallbladder. I. Circuit analysis and steady state effects of mucosal solution ionic substitutions. J Membr Biol 25:115–139
Sackin H, Boulpaep EL (1981) Isolated perfused salamander proximal tubule: methods, lectrophysiology, and transport. Am J Physiol 241:F39-F52
Ullrich KJ, Frömter E, Baumann K (1969) Micropuncture and microanalysis in kidney physiology. In: Passow H, Stämpfli R (eds) Laboratory techniques in membrane biophysics. Springer, Berlin Heidelberg New York, pp 106–129
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. T. Hoshi, Dept. of Physiology, University of Tokyo, Japan, a pioneer in renal tubular resistance analysis
This work was supported by the Deutsche Forschungsgemeinschaft
Rights and permissions
About this article
Cite this article
Cook, D.I., Frömter, E. Is the voltage divider ratio a reliable estimate of the resistance ratio of the cell membranes in tubular epithelia?. Pflugers Arch. 403, 388–395 (1985). https://doi.org/10.1007/BF00589251
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00589251