Summary
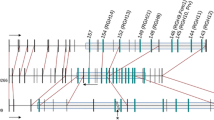
The sequences of the genes coding for a hydroxyproline-rich glycoprotein from two varieties of maize (Zea mays, Ac1503 and W22), a teosinte (Zea diploperennis) and sorghum (Sorghum vulgare) have been obtained and compared. Distinct patterns of variability have been observed along their sequences. The 500 by region immediately upstream of the TATA box is highly conserved in theZea species and contains stretches of sequences also found in the sorghum gene. Further upstream, significant rearrangements are observed, even between the two maize varieties. These observations allow definition of a 5′ region, which is common to the four genes and is probably essential for their expression. The 3′ end shows variability, mostly due to small duplications and single nucleotide substitutions. There is an intron present in this region showing a high degree of sequence conservation among the four genes analyzed. The coding region is the most divergent, but variability arises from duplications of fragments coding for similar protein blocks and from single nucleotide substitutions. These results indicate that a number of distinct mechanisms (probably point mutation, transposon insertion and excision, homologous recombination and unequal crossing-over) are active in the production of sequence variability in maize and related species. They are revealed in different parts of the gene, probably as the result of the different types of functional constraints acting on them, and of the specific nature of the sequence in each region.
Similar content being viewed by others
References
Broglie KE, Biddle P, Cressman R, Broglie R (1989) Functional analysis of DNA sequences responsible for ethylene regulation of a bean chitinase gene in transgenic tobacco. Plant Cell 1:599–607
Burr B, Burr FA (1981) Controlling-element events at the shrunken locus in maize. Genetics 98:143–156
Chen JA, Varner JE (1985) An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J 4:2145–2151
Dixon LK, Hohn T (1984) Initiation of translation of the cauliflower mosaic virus genome from a polycistronic mRNA: evidence from deletion mutagenesis. EMBO J 3:2731–2736
Dover GA (1986) Molecular drive in multigene families: how biological novelties arise spread and are assimilated. Trends Genet 2:159–165
Efstratiadis A, Posakony JW, Maniatis T, Lawn RM, O'Connell C, Spritz RA, DeRiel JK, Forget BG, Weissman SM, Slightom JL, Blechl AE, Smithies O, Baralle FE, Shoulders CC, Proudfoot NJ (1980) The structure and evolution of the human β-globin gene family. Cell 21:653–658
Fedoroff NV (1989) About maize transposable elements and development. Cell 56:181–191
Felsenstein J (1990) PHYLIP Manual. University Herbarium of the University of California, Berkeley, Calif
Keller B, Lamb CJ (1989) Specific expression of a novel cell wall hydroxyproline-rich glycoprotein gene in lateral root initiation. Genes Dev 3:1639–1646
Kieliszewski MJ, Leykam JF, Lamport DTA (1990) Structure of the threonine-rich extensin from Zea mays. Plant Physiol 92:316–326
Li W-H, Luo C-C, Wu C-I (1985a) Evolution of DNA sequences. In: MacIntyre RJ (ed) Molecular Evolutionary Genetics. Plenum Press, New York pp 65–76
Li, W-H, Wu C-I, Luo C-C (1985b) A new method for estimating synonymous and nonsynonymous rates of nucleotide substitution considering the relative likelihood of nucleotide and codon changes. Mol Biol Evol 2:150–174
Loenen WAM, Blattner FR (1983) Lambda Charon vectors (Ch 32, 33, 34 and 35) adapted for DNA cloning in recombination-deficient hosts. Gene 26:171–179
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press Cold Spring Harbor, NY
Maxam AM, Gilbert W (1980) Sequencing end-labelled DNA with base-specific chemical cleavages. Methods Enzymol 65:499–560
McClintock B (1951) Chromosome organization and genic expression. Cold Spring Harbor Symp Quant Biol 16:13–47
Prat S, Perez-Grau L, Puigdomènech P (1987) Multiple variability in the sequence of a family of maize endosperm proteins. Gene 52:41–49
Queen C, Korn LJ (1984) A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res 12:581–599
Raz R, Crétin C, Puigdomènech P, Martínez-Izquierdo JA (1991) The sequence of a hydroxyproline-rich glycoprotein gene from Sorghum vulgare. Plant Mol Biol 16:365–367
Ruiz-Carrillo A, Renaud J (1987) Endonuclease G: a (dG)n · (dC)n specific DNase from higher eukaryotes. EMBO J 6:401–407
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-termination inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schmid CW, Shen C-KJ (1985) The evolution of interspersed repetitive DNA sequences in mammals and other vertebrates. In: MacIntyre RJ (ed) Molecular Evolutionary Genetics. Plenum Press, New York pp 323–358
Schwarz-Sommer Z, Gierl A, Cuypers H, Peterson PA, Saedler H (1985) Plant transposable elements generate the DNA sequence diversity needed in evolution. EMBO J 4:591–597
Showalter AM, Bell JN, Cramer CL, Bailey JA, Varner JE, Lamb CJ (1985) Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci USA 82:6551–6555
Stiefel V, Pérez-Grau L, Albericio F, Giralt E, Ruiz-Avila L, Ludevid D, Puigdomènech P (1988) Molecular cloning of cDNAs encoding a putative cell wall protein from Zea mays and immunological identification of related polypeptides. Plant Mol Biol 11:483–493
Stiefel V, Ruiz-Avila L, Raz R, Vallés MP, Gómez J, Pagés M, Martínez-Izquierdo JA, Ludevid D, Langdale JA, Nelson T, Puigdomènech P (1990) Expression of a maize cell wall hydroxyproline-rich glycoprotein gene in early leaf and root vascular differentiation. Plant Cell 2:785–793
Tajima F, Nei M (1984) Estimation of evolutionary distance between nucleotide sequences. Mol Biol Evol 1:269–285
Author information
Authors and Affiliations
Additional information
Communicated by H. Saedler
The sequences reported in this paper have been deposited in the EMBL/GenBank Database (Bolt, Beranek, and Newman Laboratories, Cambridge, Mass., and EMBL, Heidelberg), accession nos. M36635 (maize Ac1503), X63134 (maize W22), X64173 (teosinte) and X56010 (sorghum)
Rights and permissions
About this article
Cite this article
Raze, R., José, M., Moya, A. et al. Different mechanisms generating sequence variability are revealed in distinct regions of the hydroxyproline-rich glycoprotein gene from maize and related specie*. Molec. Gen. Genet. 233, 252–259 (1992). https://doi.org/10.1007/BF00587586
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00587586