Summary
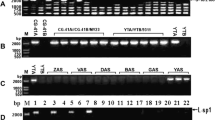
ANicotiana sylvestris plant regenerated from protoplast culture was found to be mutated in both the mitochondrial (mt) and nuclear genomes. The novel mt DNA organization, called U, is due to the amplification of recombinant substoichiometric DNA sequences that preexist in the parent line. The recombination event involves two 404 by repeats, which hybridize to a 2.1 kb transcript. Although the sequence of both repeats was not altered by the recombination, an additional transcript of 2.5 kb was detected in U mitochondria. In addition to this mitochondrial reorganization, the protoclone carried a recessive nuclear mutation conferring male sterility (ms4). A possible role ofms4 in the appearance of the U mt DNA organization was investigated by introducing this gene into normalN. sylvestris cytoplasm. No mt DNA change could be found in homozygousms4/ms4 plants of the F2 generation.
Similar content being viewed by others
Abbreviations
- mst:
-
nuclear male sterile
- CNS:
-
cytoplasmic male sterile
- mt:
-
mitochondrial
- ct:
-
chloroplast
References
Alexander MP (1969) Differential staining of aborted and non aborted pollen. Stain Technol 44:117–122
Bendich AJ, Smith SB (1990) Moving pictures and pulse-field gel electrophoresis show linear DNA molecules from chloroplasts and mitochondria. Curr Genet 17:421–426
Brears T, Curtis GJ, Lonsdale DM (1989) A specific rearrangement of mitochondrial DNA induced by tissue culture. Theor Appl Genet 77:620–624
Chétrit P, Gaudin V, de Courcel A, Vedel F (1989) A cross hybridization method for DNA mapping with photobiotin-labeled probes. Anal Biochem 178:273–275
Chétrit P, Rios R, De Paepe R, Vitart V, Gutierres S, Vedel F (1992) Cytoplasmic male sterility is associated with large deletions in the mitochondrial DNA of twoNicotiana sylvestris protoclones. Curr Genet 21:131–137
Dale RMK, Duessing JH, Keene D (1981) Supercoiled mitochondrial DNAs from plant tissue culture cells. ucleic Acids Res 9:4583–4593
De Paepe R, Chetrit P, Vitart V, Ambard-Bretteville F, Prat D, Vedel F (1990) Several nuclear genes control both male sterility and mitochondrial protein synthesis inNicotiana sylvestris protoclones. Mol Gen Genet 222:206–210
Escote-Carlson LJ, Gabay-Laughnan S, Laughnan JR (1990) Nuclear genotype affects mitochondrial genome organization of CMS-S maize. Mol Gen Genet 223:457–464
Hartmann C, Henry Y, De Buyser J, Aubry C, Rode A (1989) Identification of new mitochondrial genome organizations in wheat plants regenerated from somatic tissue cultures. Theor Appl Genet 77:169–175
Kimura M, Kimura J, Watanabe K (1985) The primary structure of ribosomal protein L2 fromBacillus stearothermophilus. Eur J Biochem 153:289–297
Li XQ, Chétrit P, Mathieu C, Vedel F, De Paepe R, Rémy R, Ambard-Bretteville F (1988) Regeneration of cytoplasmic male sterile protoclones ofNicotiana sylvestris with mitochondrial variations. Curr Genet 13:261–266
Lonsdale DM, Hodge TP, Fauron CMR (1984) The physical map and organization of the mitochondrial genome from the fertile cytoplasm of maize. Nucleic Acids Res 12:9249–9261
Mackenzie SA, Chase CD (1990) Fertility restoration is associated with loss of a portion of the mitochondrial genome in cytoplasmic male-sterile common bean. Plant Cell 2:905–912
Mulligan RM, Lau GT, Walbot V (1988) Numerous transcription initiation sites exist for the maize mitochondrial genes for subunit 9 of the ATP synthase and subunit 3 of cytochrome oxidase. Proc Natl Acad Sci USA 85:7998–8002
Newton KJ, Knudsen C, Gabay-Laughnan S, Laughnan JR (1990) An abnormal growth mutant in maize has a defective mitochondrial cytochrome oxidase gene. Plant Cell 2:107–113
Palmer JD, Shields CR (1984) Tripartite structure of theBrassica campestris mitochondrial genome. Nature 307:437–440
Pearson WR, Lipman DJ (1988) Improved tools for biological sequence comparison. Proc Natl Acad Sci USA 85:2444–2448
Prat D (1983) Genetic variability induced inN. sylvestris by protoplast culture. Theor Appl Genet 64:223–230
Rottman WH, Brears T, Hodge TP, Lonsdale DM (1987) A mitochondrial gene is lost via homologous recombination during reversion of CMS-T maize to fertility. EMBO J 6:1541–1546
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
San HL, Vedel F, Sihachakr D, Rémy R (1990) Morphological and molecular characterization of fertile tetraploid somatic hybrids produced by protoplast electrofusion and PEG-induced fusion betweenLycopersicon esculentum Mill. andLycopersicon peruvianum Mill. Mol Gen Genet 221:17–26
Sanger DB, Nicklen S, Coulsen AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Shinozaki K, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsubayashi T, Zaita N, Chunwongse J, Obokata J, Yamaguchi-Shinozaki K, Ohto C, Torazaura K, Meng BY, Sugita M, Deno H, Kamogashira T, Yamada K, Kusuda J, Takaiwa F, Kato A, Tohdoh N, Shimada H, Sugiura M (1986) The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J 5:2043–2049
Shirzadegan M, Christey M, Earle ED, Palmer JD (1989) Rearrangement, amplification and assortment of mitochondrial DNA molecules in cultured cells ofBrassica campestris. Theor Appl Genet 77:17–25
Small ID, Isaac PG, Leaver CJ (1987) Stoichiometric differences in DNA molecules containing theatpA gene suggest mechanisms for the generation of mitochondrial diversity in maize. EMBO J 6:865–869
Small ID, Suffolk R, Leaver CJ (1989) Evolution of plant mitochondrial genomes via substoichiometric intermediates. Cell 58:69–76
Stern DB, Lonsdale DM (1982) Mitochondrial and chloroplast genomes of maize have a 12 kilobase DNA sequence in common. Nature 299:698–702
Stern DB, Newton KJ (1986) Isolation of plant mitochondrial RNA. Methods Enzymol 118:488–496
Wintz M, Chen MC, Pillay DTN (1988) Presence of a chloroplastlike elongator tRNAMet gene in the mitochondrial genomes of soybean andArabidopsis thaliana. Curr Genet 13:255–260
Zhu J, Kempenaers W, Van der Straeten D, Contreras R, Fiers W (1985) A method for fast and pure DNA elution from agarose gels by centrifugal filtration. Biotechnology 3:1014–1016
Author information
Authors and Affiliations
Additional information
Communicated by R. Herrmann
Rights and permissions
About this article
Cite this article
Vitart, V., De Paepe, R., Mathieu, C. et al. Amplification of substoichiometric recombinant mitochondrial DNA sequences in a nuclear, male sterile mutant regenerated from protoplast culture inNicotiana sylvestris . Molec. Gen. Genet. 233, 193–200 (1992). https://doi.org/10.1007/BF00587579
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00587579