Summary
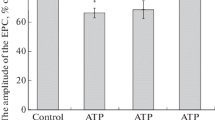
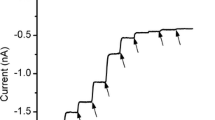
Incubation of the hypotrichous ciliateStylonychia mytilus in fluorescein-labeled concanavalin A (Con A, 0.1–0.5 μg/ml) produced a strong fluorescence of its membranelles, but comparatively weak fluorescence of the other compound cilia and of the somatic membrane. Compared to untreated cells, the frequency of spontaneous backward movements was reduced in the presence of 0.5 μg/ml ConA. In electrophysiological experiments Con A altered the excitability of the cell membrane. The two-peak action potential lost its second component which is associated with voltage-dependent Ca channels in the membranelles. The corresponding Ca current (Ca current I) was inhibited by low concentrations of Con A (0.2–0.5 μg/ml). A second voltage-dependent Ca current (Ca current II) was not affected. Reducing the K outward current by intracellular Cs and/or extracellular tetraethylammonium, or changing the holding potential, did not restore the Con A-sensitive Ca current I. Con A also inhibited this current when Ca was replaced by Ba. The inhibitory effect of Con A on the voltagedependent Ca current I was prevented by 10–30 mM α-methyl-d-mannoside, and the lectin wheat germ agglutinin (20 μg/ml) did not affect the Ca currents, indicating that the Con A effect was mediated by binding to specific sugar residues on the excitable membrane. The succinylated dimeric derivative of Con A did not inhibit Ca current I up to concentrations of 5 μg/ml. It is concluded that the two voltage-dependent Ca currents inStylonychia can be chemically isolated due to their different sensitivity to Con A, which appears to bind preferentially to sites near or at the Ca channel in the membranellar membrane.
Similar content being viewed by others
References
Ballanyi K, Deitmer JW (1984) Concentration-dependent effects of Ba on action potential and membrane currents in the ciliateStylonychia. Comp Biochem Physiol 78A:575–581
Borsotto M, Barhanin J, Norman RI Lazdunski M (1984) Purification of the dihydropyridine receptor of the voltage-dependent Ca channel from skeletal muscle transverse tubules using (+)-[3H]-PN 200-110. Biochem Biophys Res Comm 122:1357–1366
Bourguignon LYW, Bourguignon GJ (1984) Capping and the cytoskeleton. Int Rev Cytol 87:1195–1224
Cameron AR, Nelson J, Forman HJ (1983) Depolarization and increased conductance precede superoxide release by concanavalin A-stimulated rat alveolar macrophages. Proc Natl Acad Sci USA 80:3726–3728
Curtis BM, Catteral WA (1983) Solubilization of the calcium antagonist receptor from rat brain. J Biol Chem 258:7280–7283
Deitmer JW (1982) Two voltage-dependent calcium conductances in the membrane of the ciliateStylonychia. Pflügers Arch Suppl 392:R20
Deitmer JW (1983) Ca channels in the membrane of the hypotrich ciliateStylonychia. In: Grinnel AD, Moody WJ (eds) The physiology of excitable cells. AR Liss Inc, New York, pp 51–63
Deitmer JW (1984) Evidence for two voltage-dependent calcium currents in the membrane of the ciliateStylonychia. J Physiol (Lond) 355:137–159
Deitmer JW, Machemer H, Martinac B (1984) Motor control in three types of ciliary organelles ofStylonychia. J Comp Physiol 154:113–124
Dudel J (1979) The voltage dependence of the decay of the excitatory postsynaptic current and the effect of concanavalin A at the crayfish neuromuscular junction. J Physiol (Paris) 75:601–604
Eckert R (1972) Bioelectric control of ciliary activity. Science 76:473–481
Edelman GM, Yahara I, Wang JL (1973) Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci USA 70:1442–1446
Fishman MC, Dragsten PR, Spector I (1981) Immobilization of concanavalin A receptors during differentiation of neuroblastoma cells. Nature 290:781–783
Frisch A, Bessler W, Lipps JH, Ammermann D (1976) Immobilization of ciliates by concanavalin A. J Protozool 23:427–430
Goldstein IJ, Hollerman CE, Merrick JM (1965) Protein-carbohydrate interaction I. The interaction of polysaccharides with concanavalin A. Biochim Biophys Acta 97:68–76
Grimes GW (1982) Pattern determination in hypotrich ciliates. Am Zool 22:35–46
Gunther GR, Wang JL, Yahara I, Cunningham BA, Edelman GM, (1973) Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci USA 70:1012–1016
Hagiwara S, Byerly L (1981) The calcium channel. Annu Rev Neurosci 4:69–125
Ivens I, Dietmer JW (1984) Inhibition of calcium currents by Concanavalin A. Pflügers Arch Suppl 402:R34
Lis H, Sharon N (1973) The biochemistry, of plant lectins (Phytohemagglutinins). Annu Rev Biochem 42:541–574
Machemer H (1970) Korrelation zwischen Membranpotential und fortbewegung beiStylonychia. Naturwissenschaften 57:398–399
Machemer H, de Peyer JE (1977) Swimming sensory cells: electrical membrane parameters, receptor properties, and motor control in ciliated protozoa. Verh Dtsch Zool Ges (1977) 86–110
Mathers DA (1981) The influence of concanavalin A on tamate-induced current fluctuations in locust muscle fibers. J Physiol (Lond) 312:1–8
Mathers DA, Usherwood PNR (1976) Concanavalin A blocks desensitisation of glutamate receptors on insect muscle fibres. Nature 259:401–411
Myrdal SM, DeHaan RL (1983) Concanavalin A increases spontaneous beat rate of embryonic chick heart cell aggregates. J Cell Physiol 117:319–325
Nicolson GL (1974) The interactions of lectins with animal cell surfaces. Int Rev Cytol 39:89–190
Ozaro K, Huang L, Ebert JD (1977) Accelerated calcium ion uptake in murine thymocytes induced by concanavalin A. J Cell Physiol 93:153–160
de Peyer JE, Deitmer JW (1980) Divalent cations as charge carriers during two functionally different membrane currents in the ciliateStylonychia. J Exp Biol 88:73–89
de Peyer JE, Machemer H (1977) Membrane excitability inStylonychia: properties of the two-peak regenerative Ca-response. J Comp Physiol 121:15–32
Sharon N, Lis H (1972) Lectins: cell-agglutinating and sugarspecific proteins. Science 177:949–959
Thieffry M (1982) Concanavalin A blocks the Ca-dependence of crayfish muscle fiber responses to glutamate. Brain Res 243:165–168
Thieffry M (1984) The effect of calcium ions on the glutamate response and its desensitization in crayfish muscle fibers. J Physiol 355:119–135
Uzgiris EE, Lockwood SH, Kaplan JH (1982) Oscillation of cell surface charge of human peripheral blood lymphocytes after stimulation with concanavalin A. J Immunol 128:1975–1978
Yahara I, Edelman GM (1972) Restriction of the mobility of lymphocyte immunoglobulin receptors by concanavalin A. Proc. Natl Acad Sci USA 69:608–612
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ivens, I., Deitmer, J.W. Inhibition of a voltage-dependent Ca current by concanavalin A. Pflugers Arch. 406, 212–217 (1986). https://doi.org/10.1007/BF00586685
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00586685