Abstract
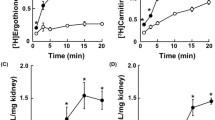
Isolated segments of cortical thick ascending limbs (cTAL) of rabbit kidney were perfused in vitro and the equivalent short circuit current (Isc) was measured. In a first series all substrates were removed on either side. Isc fell rapidly to 50±12% after 3 min and to 27±6% (n=5) after 10 min. This indicates that in cTAL segments Isc is strictly dependent on the presence of substrates. In series two it was tested what substrates can be utilized by the cTAL segment, and from which epithelial side [bath (b) or lumen (l)] the substrates are taken up. From the l-side only butyrate (10 mmol · l−1) sustained the Isc at 95±2% (n=7). All other tested substrates (10 mmol · l−1): pyruvate, acetate, β-OH-butyrate,d-glucose, andl-lactate lead to a marked decline in Isc. From the b-side several substrates (5–10 mmol · l−1) sustained the Isc:d-glucose,d-mannose, butyrate, β-OH-butyrate, acetoacetate,l-lactate, acetate and pyruvate. Other compounds (1–10 mmol · l−1): citrate, α-ketoglutarate, succinate, glutamate, glutamine, propionate, caprylate and oleate did not sustain Isc. In the third series the mechanism of substrate utilization from the basolateral cell side was studied. It was shown that the Isc is a saturable function of thed-glucose,l-lactate, acetate, pyruvate or β-OH-butyrate concentration with apparentK m's between 0.05–1.0 mmol · l−1. Several known inhibitors of sugar and of anion transport were tested at the bath side: phlorrhizin was without effect. Phloretin (500 μmol · l−1) inhibited Isc by 96%, yet its effect was not dependent on the presence of substrates on the b-side since inhibition ocurred also if the b-perfusate contained no substrate and Isc was driven by luminal butyrate. Also SITS (5 mmol · l−1) exerted only a small inhibitory effect which was not specific since it was also observed with luminal butyrate. α-Cyano-m-OH-cinnamate (10 mmol · l−1) inhibited the Isc specifically whenl-lactate was the bath substrate. Probenecid (1 mmol · l−1) had a similar yet less marked inhibitory effect. Thed-glucose uptake from the b-side was specifically inhibited by cytochalasin B at 5 · 10−6 mol · l−1. We conclude that the cTAL segment of the rabbit utilizesd-glucose and/or small anions such as pyruvate orl-lactate or acetate to energize salt reabsorption. The link between substrate availability and salt reabsorption is extremely close in this nephron segment. Substrate uptake occurs from the blood side. Sugar uptake can be inhibited by cytochalasin B andl-lactate uptake by probenecid and α-cyano-m-OH-cinnamat. These data suggest that substrate uptake at the basolateral cell side occurs probably via carrier systems.
Similar content being viewed by others
References
Andersen OS, Finkelstein A, Katz I, Cass A (1976) Effect of phloretin on the permeability in thin lipid membranes. J Gen Physiol 67:749–771
Baeyer H v (1981) Transport ofd-glucose in the mammalian kidney. In: Greger R, Lang F, Silbernagl S (eds) Renal transport of organic substances. Springer, Berlin Heidelberg New York, pp 154–177
Barac-Nieto M, Murer H, Kinne R (1982) Asymmetry in the transport of lactate by basolateral and brush border membranes of rat kidney cortex. Pflügers Arch 392:366–371
Burg MB, Grantham J, Abramow M, Orloff J (1966) Preparation and study of fragments of single rabbit nephrons. Am J Physiol 210 (6):1293–1298
Chance B (1980) Non-invasive approaches to the biomedical basis for physiological function. Proc Int Union Physiol Sci 14:14–15
Cohen JJ, Barac-Nieto M (1973) Renal metabolism of substrates in relation to renal function. In: Orloff J, Berliner RW, Geiger SR (eds) Handbook of physiology: Renal physiology. Am Physiol Soc, Washington DC, pp 909–1001
Cohen JJ, Kamm DE (1976) Renal metabolism: Relation to renal function. In: Brenner BM, Rector FC Jr (eds) The kidney. Saunders Comp, Philadelphia London Toronto, pp 126–214
De Jonge PC, Wieringa T, Putten JPM Van, Krans HMJ, Dam K Van (1983) Phloretin — an uncoupler and an inhibitor of mitochondrial oxidative phosphorylation. Biochim Biophys Acta 722:219–225
Eveloff J, Bayerdörffer, Silva P, Kinne R (1981) Sodium-chloride transport in the thick ascending limb of Henle's loop. Oxygen consumption studies in isolated cells. Pflügers Arch 389:263–270
Forman SA, Verkman AS, Dix JA, Solomon AK (1982) Interaction of phloretin with the anion transport protein of the red blood cell membrane. Biochim Biophys Acta 689:531–538
Frömter E (1982) Electrophysiological analysis of rat renal sugar and amino acid transport. I. Basic phenomena. Pflügers Arch 393:179–189
Greger R (1981a) Cation selectivity of the isolated perfused cortical thick ascending limb of Henle's loop of rabbit kidney. Pflügers Arch 390:30–37
Greger R (1981b) Coupled transport of Na+ and Cl− in the thick ascending limb of Henle's loop of rabbit kidney. In: Klinke R, Lahn W, Querfurth J, Scholtholt J (eds) Scand Audiol Suppl 14:1–15
Greger R, Hampel W (1981) A modified system for in vitro perfusion of isoated renal tubules. Pflügers Arch 389:175–176
Greger R, Schlatter E (1983a) Properties of the basolateral membrane of the cortical thick ascending limb of Henle's loop of rabbit kidney. A model for secondary active chloride transport. Pflügers Arch 396:325–334
Greger R, Schlatter E (1983b) Cellular mechanism of the action of loop diuretics on the thick ascending limb of Henle's loop. Klin Wochenschr 61:1019–1027
Greger R, Schlatter E (1983c) Properties of the lumen membrane of the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflügers Arch 396:315–324
Griffin JF, Rampal AL, Jung CH (1982) Inhibition of glucose transport in human erythrocytes by cytochalasins: A model based on diffraction studies. Proc Natl Acad Sci USA 79: 3759–3763
Guder WG, Schmidt U (1976) Substrate and oxygen dependence of renal metabolism. Kidney Int 10:32–38
Guder WG, Wirthensohn G (1981) Renal turnover of substrates. In: Greger R, Lang F, Silbernagl S (eds) Renal transport of organic substances. Springer, Berlin Heidelberg New York, pp 65–77
Guder WG, Ross BD (1984) Enzyme distribution along the nephron. Kidney Int (in press)
Häberle DA (1981) Characteristics of p-aminohippurate transport in the mammalian kidney. In: Greger R, Lang F, Silbernagl S (eds) Renal transport of organic substances. Springer, Berlin Heidelberg New York, pp 189–209
Hebert SC, Culpepper RM, Andreoli TE (1981) NaCl transport in mouse medullary thick ascending limbs. I. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. Am J Physiol 241:F412-F431
Hopfer U, Sigrist-Nelson K, Amman E, Murer H (1976) Differences in neutral amino acid and glucose transport between brush border and basolateral plasma membrane of intestinal epithelial cells. J Cell Physiol 89:805–810
Höhmann B, Zwiebel R, Yamagata A, Kinne R (1969) Enzymaktivitäten im isolierten proximalen Tubulus der Kaninchenniere. Pflügers Arch 312:110–125
Kalckar H (1936) Inhibitory effect of phloridzin and phloretin on kidney phosphatase. Nature 138:289
Kimmich GA, Randles J (1975) A Na+-independent, phloretin-sensitive monosaccharide transport system in isolated intestinal epithelial cells. J Membr Biol 23:57–76
Kinne R, Murer H (1976) Polarity of epithelial cells in relation to transepithelial transport in kidney and intestine. In: Robinson JWL (ed) Intestinal ion transport. MTP Press Ltd., Lancaster, pp 79–95
Kinne R, Murer H, Kinne-Saffran E, Thees M, Sachs G (1975) Sugar transport by renal plasma membrane vesicles. Characterization of the systems in the Brush-Border Microvilli and basal-lateral plasma membranes. J Membr Biol 21:375–395
Klein KL, Wang M-S, Torikai S, Davidson WD, Kurokawa K (1981) Substrate oxidation by isolated single nephron segments of the rat. Kidney Int 20:29–35
Kurokawa K, Torikai S, Wang M-S, Klein KL (1982) Metabolic heterogeneity of the nephron. Mineral Electrolyte Metab 77:225–236
Le Bouffant F, Hus-Citharel A, Morel F (1982) In vitro14CO2 production by single pieces of rat cortical thick ascending limbs and its coupling to active salt transport. In: Morel F (ed) Biochemistry of kidney functions, INSERM Symposium 21. Elsevier Biomedical Press, Amsterdam New York Oxford, pp 363–370
LeFevre PG, Marshall JK (1959) The attachment of phloretin and analogues to human erythrocytes in connection with inhibition of sugar transport. J Biol Chem 234 (11):3022–3026
Le Hir M, Dubach UC (1982) Activities of enzymes of the tricarboxylic acid cycle in segments of the rat nephron. Pflügers Arch 395:239–243
Macey RI, Farmer REL (1970) Inhibition of water and solute permeability in human red cells. Biochim Biophys Acta 211:104–106
Murer H, Barac-Nieto M, Ullrich KJ, Kinne R (1981) Renal transport of lactate. In: Greger R, Lang F, Silbernagl S (eds) Renal transport of organic substances. Springer, Berlin Heidelberg New York, pp 210–223
Murer H, Kinne R (1977) Sidedness and coupling of transport in small intestinal and renal epithelia. In: Semenza G, Carafoli E (eds) Biochemistry of membrane transport, FEBS-Symposium No. 42. Springer, Berlin Heidelberg New York, pp 292–304
Owen JD (1974) The effect of phloretin on the potassium conductance in Aplysia giant neurons. J Membr Biol 16:65–78
Owen JD, Solomon AK (1972) Control of nonelectrolyte permeability in red cells. Biochim Biophys Acta 290:414–418
Owen A, Caplan SR, Essig A (1975) A comparison of the effects of ouabain and 2-deoxy-d-glucose on the thermodynamic variables of the frog skin. Biochim Biophys Acta 394:438–448
Petersen K-U, Wood JR, Schulze G, Heintze K (1981) Stimulation of gallbladder fluid and electrolyte absorption by butyrate. J Membr Biol 62:183–193
Randles J, Kimmich GA (1978) Effects of phloretin and theophylline on 3-O-methyl-glucose transport by intestinal epithelial cells. Am J Physiol 234 (3):C64-C72
Reyes J, Greco F, Otais R, Latorre R (1983) Phloretin and phloretin analogs: Mode of action in planar lipid bilayers and monolayers. J Membr Biol 72:93–103
Schlatter E, Greger R (1982) Metabolic substrates for maintaining active NaCl transport in the isolated cortical thick ascending limb (cTAL) of rabbit kidney. Pflügers Arch 394:R22
Schmidt I, Guder WG (1976) Sites of enzyme activity along the nephron. Kidney Int 9:233–242
Shimada H, Endou H, Sakai F (1982) Distribution of α-glutamyl transpeptidase and glutaminase isoenzymes in the rabbit single nephron. Jpn J Pharmacol 32:121–129
Silverman M, Turner RJ (1982) 2-Deoxy-d-glucose transport in dog kidney. Am J Physiol 242:F711-F720
Ullrich KJ (1976) Renal tubular mechanisms of organic solute transport. Kidney Int 9:134–148
Ullrich KJ (1979) Sugar, amino acid, and Na+ cotransport in the proximal tubule. Ann Rev Physiol 41:181–195
Ullrich KJ, Fasold H, Rumrich G, Klöss S (1984) Secretion and contraluminal uptake of dicarboxylic acids in the proximal convolution of rat kidney. Pflügers Arch 400:241–249
Ullrich KJ, Radtke HW, Rumrich G (1971) The role of bicarbonate and other buffers on isotonic fluid absorption in the proximal convolution of the rat kidney. Pflügers Arch 330:149–161
Ullrich KJ, Rumrich G, Klöss S (1982) Reabsorption of monocarboxylic acids in the proximal tubule of the rat kidney. I. Transport kinetics ofd-lactate, Na+-dependence, pH-dependence and effect of inhibitors. Pflügers Arch 395:212–219
Ullrich KJ, Rumrich G, Klöss S, Fasold H (1982) Reabsorption of monocarboxylic acid in the proximal tubule of the rat kidney. III. Specificity for aromatic compounds. Pflügers Arch 395:227–231
Vandewalle A, Wirthensohn G, Heidrich H-G, Guder WG (1981) Distribution of hexokinase and phosphoenolpyruvate carboxykinase along the rabbit nephron. Am J Physiol 240:F492-F500
Warnock DG, Greger R, Dunham PB, Frizzell RA, Field M, Spring KR, Ives HE, Aronsen PS, Seifter J (1984) Ion transport processes in apical membranes of epithelia. Fed Proc (in press)
Wright EM (1974) Active transport of iodide and other anions across the choroid plexus. J Physiol 240:535–566
Wright EM, Van Os CH, Mircheff AK (1980) Sugar uptake by intestinal basolateral membrane vesicles. Biochim Biophys Acta 597:112–124
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wittner, M., Weidtke, C., Schlatter, E. et al. Substrate utilization in the isolated perfused cortical thick ascending limb of rabbit nephron. Pflugers Arch. 402, 52–62 (1984). https://doi.org/10.1007/BF00584832
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00584832