Abstract
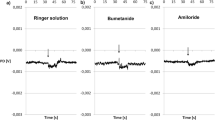
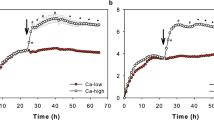
Structural and functional changes induced by long-term Li-exposure of the outer surface of toad skin was studied. Electron microscopy revealed that total Na by Li replacement in the outer compartment of short-circuited toad skin promotes a conspicuous cellular damage expressed as focal swollen cells with altered intercellular spaces and nuclear morphology. Short-circuit current (SCC) decreases by about 70% over the first 60 min after 115 mmol/l Li-exposure. An amiloride sensitive transepithelial Li transport remains intact over a further 150 min despite the epithelial damage, indicating that the pathways across the apical barrier are functioning. Increase of the paracellular permeability is detected by elevation of Na-efflux. Partial 50% or 10% Na by Li replacements induce minor structural alterations and are not sufficient to trigger appreciable Na-efflux and SCC alterations. Therefore, low Li concentrations are less effective than 115 mmol/l in promoting morphofunctional responses. Although ouabain and Li reduce the SCC, ouabain does not promote structural lesions, showing that Li-inhibition of Na transport by itself is not responsible for the observed morphological alterations. In the light of this study, Li utilization as a tool to investigate transepithelial Na transport requires careful judgement.
Similar content being viewed by others
References
Aboulafia J, Sanioto SML, Lacaz-Vieira F (1983) Cellular Li opens paracellular path in toad skin: amiloride blockable effect. J Membr Biol 74:59–65
Aurell M, Svalander C, Allin L, Alling C (1981) Renal function and biopsy findings in patients on long-term lithium treatment. Kidney Int 20:663–670
Benos DJ, Mandel LJ, Simon SA (1980) Cationic selectivity and competition at the sodium entry site in frog skin. J Gen Physiol 76:233–247
Bevevino LH, Lacaz-Vieira F (1982) Control of sodium permeability of the outer barrier in toad skin. J Membr Biol 66:97–107
Biber TUL, Curran PF (1970) Direct measurement of uptake of sodium at the outer surface of the frog skin. J Gen Physiol 56:83–99
Candia OA, Chiarandini DJ (1973) Transport of lithium and rectification by frog skin. Biochim Biophys Acta 307:578–589
Christensen S, Hansen BB, Faarup P (1982) Functional and structural changes in the rat kidney by long-term lithium treatment. Renal Physiol 5:95–104
Evan AP, Ollerick DA (1972) The effect of lithium carbonate on the structure of the rat kidney. Am J Anat 134:97–106
Fernandez-Repollet E, LeFurgey A, Hardy MA, Tisher CC (1983) Structural and functional response of toad urinary bladder to LiCl. Kidney Int 24:719–730
Forrest JM Jr, Cohen AD, Torretti J, Himmelhoch JM, Epstein FH (1974) On the mechanism of lithium-induced diabetis insipidus in man and rat. J Clin Invest 53:1115–1123
Forrest JM Jr, Marcy TW, Biemesderfer D, Kashgarian M (1981) Cytoskeletal defect in cortical collecting duct cells in lithium-induced polyuria. Kidney Int 19:200
Gutman Y, Hochman S, Wald H (1973) The differential effect of Li on microsomal ATPase in cortex, medulla and papilla of the rat kidney. Biochim Biophys Acta 298:284–290
Hansen HH, Zerahn K (1964) Concentration of lithium, sodium and potassium in epithelial cells of the isolated frog skin during active transport of lithium. Acta Physiol Scand 60:189–196
Hestbech J, Hansen HE, Amdisen A, Olsen S (1977) Chronic renal lesions following long-term treatment with lithium. Kidney Int 12:205–213
Hestbech J, Olesen OV, Thomsen K (1978) Lithium-induced focal interstitial fibrosis in rat kidney. Acta Pathol Microbiol Immunol Scand 36:195–197
Hughes MP, Macknight ADC (1982) Cellular lithium and transepithelial transport across toad urinary bladder. J Membr Biol 70:69–88
Hullin RP (1975) The effects of lithium on electrolyte balance and body fluids. In: Johnson FM (ed) Lithium research and therapy. Academic Press, London, pp 359–379
Jørgensen F, Larsen S, Spanager B, Clausen E, Tango M, Brinch E, Brun C (1984) Kidney function and quantitative histological changes in patients on long-term lithium therapy. Acta Psychiatr Scand 70:455–462
Kling MA, Fox JG, Johnston SM, Tolkoff-Rubbin NE, Rubin RH, Colvin R (1984) Effects of long-term lithium administration on renal structure and function in rats. A distinctive tubular lesion. Lab Invest 50:526–535
Leblanc G (1972) The mechanism of lithium accumulation in the isolated frog skin epithelium. Pflügers Arch 337:1–18
Li JHY, Lindemann B (1982) Movement of Na and Li across the apical membrane of frog skin. In: Emrich JBA, Lux HH (eds) Basic mechanisms in the action of lithium. Excerpta Medica, amsterdam, pp 23–35
MacAuliffe WG, Olesen OV (1983) Effects of lithium on the structure of the rat kidney. Nephron 34:114–124
Morel F, Leblanc G (1975) Transient current changes and Na compartmentalization in frog skin epithelium. Pflügers Arch 358:135–157
Moyer BJ (1962) A survey of Cerenkov counter technique. In: Snell AH (ed) Nuclear instruments and their uses, vol 1. Wiley and Sons, New York, pp 166–193
Nagel W (1977) Influence of lithium upon the intracellular potential of frog skin epithelium. J Membr Biol 37:347–359
Nørgaard T, Faarup P, Hansen BB, Kristensen AR, Christensen S (1985) Correlation between distal nephron enzyme activity structure and function in rats during lithium plus neuroleptic treatment. Renal Physiol 8:50–61
Reinach PS, Candia OA, Siegel GJ (1975) Lithium transport across isolated frog skin epithelium. J Membr Biol 25:75–92
Sanioto SML, Aboulafia J (1985) Lack of PCMB action upon the outer barrier sodium permeability in the absence of Na in toad skin. Pflügers Arch 403:115–119
Schou M (1958) Lithium studies. I. Toxicity. Acta Pharmacol Toxicol 15:70–84
Siegel GJ, Tormay A, Candia OA (1975) Microsomal (Na−K)-activated ATPase from frog skin epithelium: cation activations and some effects of inhibitors. Biochim Biophys Acta 389:557–566
Ussing HH (1960) The frog skin potential. J Gen Physiol 43:135–147
Varanda WA, Lacaz-Vieira F (1978) Transients in toad skin: short-circuit current and ionic fluxes related to inner sodium substitution by monovalent cations. J Membr Biol 39:369–385
Varanda WA, Lacaz-Vieira F (1979) Transient potassium fluxes in toad skin. J Membr Biol 49:199–233
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sanioto, S.M.L., Sesso, A. Structural and functional response of the isolated toad skin to mucosal lithium. Pflugers Arch. 409, 188–193 (1987). https://doi.org/10.1007/BF00584770
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00584770