Abstract
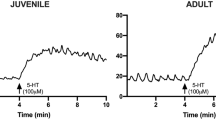
The guinea-pig vas deferens is a quiescent muscle which after castration undergoes atrophy and shows spontaneous contractions preceded by membrane spike activity. The influence of castration on the spontaneous release of neurotransmitters and on the internal concentration of sodium and potassium ions was studied. Utilizing the microelectrode technique it was shown that castration induces a partial depolarization (10 mV) of the cell membrane, but did not change the frequency of spontaneous excitatory junction potentials (SEJPs) of guinea-pig vas deferens. However, the time-course and the amplitude of the SEJPs were increased after castration, probably because of changes in membrane properties related to organ atrophy. Castration probably promotes a change in the ionic permeability of the smooth muscle fibre, since the ratio pNa/pK was twice that of control muscles.
Similar content being viewed by others
References
Aickin CC, Brading AF (1983) Towards an estimate of chloride permeability in the smooth muscle of guinea-pig vas deferens. J Physiol (Lond) 336:179–197
Aickin CC, Brading AF (1984) The role of chloride-bicarbonate exchange in the regulation of intracellular chloride in guineapig vas deferens. J Physiol (Lond) 349:587–606
Arnold AP (1981) Model systems for the study of sexual differentiation of the nervous system. Trends Pharmacol Sci 2:148–149
Blakeley AGH, Cunnane T (1979) The packed release of transmitter from the sympathetic nerves of the guinea-pig vas deferens: an electrophysiological study. J Physiol (Lond) 296:85–96
Burnstock G, Holman M (1962a) Spontaneous potentials at sympathetic nerve endings in smooth muscle. J Physiol (Lond) 160:446–460
Burnstock G, Holman M (1962b) Effect of denervation and of reserpine treatment on transmission at sympathetic nerve endings. J Physiol (Lond) 160:461–469
Casteels R (1969) Ion content and ion fluxes in the smooth muscle of the longitudinal layer of the guinea-pig's vas deferens. Pflügers Arch 313:95–105
Casteels R, Kuriyama H (1965) Membrane potential and ionic content in pregnant and non-pregnant rat myometrium. J Physiol (Lond) 177:263–287
Erulkar SD, Kelley DB, Jurman ME, Zemlan FP, Schneider GT, Krieger NR (1981) Modulation of the neural control of the clasp reflex in maleXenopus laevis by androgens: A multidisciplinary study. Proc Natl Acad Sci USA 78:5876–5880
Fatt P, Katz B (1952) Spontaneous sub-threshold activity at motor nerve endings. J Physiol (Lond) 117:109–128
Fleming WW, Westfall DP (1975) Altered resting membrane potential in the supersensitive vas deferens of the guinea-pig. J Pharmacol Exp Ther 192:381–389
Fried G, Langercrantz H, Hokfelt T (1978) Improved isolation of small noradrenergic vesicles from rats seminal ducts following castration. A density gradient and morphological study. Neuroscience 3:1271–1291
Goto K, Westfall DP, Fleming WW (1978) Denervations induced changes in electrophysiologic parameters of the smooth muscle of the guinea-pig and rat vas deferens. J Pharmacol Exp Therap 204:325–333
Markus RP, Lapa AJ, Valle JR (1980) Spontaneous contractions and membrane activity of castrated guinea-pig vas deferens. J Pharmacol Exp Therap 214:423–426
Martins T, Valle JR (1939) Endocrine control of the motility of the male accessory genital organs. Endocrinology 25:80–90
McAffee JG, Gagne G, Atkins HL, Kirchner PT, Reba RC, Blaufox MD, Smith EM (1979) Biological distribution and excretion of DTPA labeled with Tc-99 m and In-111. J Nucl Med 20:1273–1278
Merrilles CRN (1968) The nervous environment of individual smooth muscle cells of the guinea-pig vas deferens. J Cell Biol 37:794–817
Mullins LJ, Noda K (1963) The influence of sodium free solutions on the membrane potential of frog muscle fibres. J Gen Physiol 47:117–132
Souccar C, Lapa AJ, Valle JR (1982) The influence of testosterone on neuromuscular transmission of hormone sensitive mammalian skeletal muscles. Nerve Muscle 5:232–237
Snedecor GW, Cochran WG (1967) Statistical methods, 6th edn. The Iowa State University Press, Ames, pp 84–85
Urquilla PR, Westfall DP, Goto K, Fleming WW (1978) The effects of ouabain and alterations in potassium concentration on the sensitivity to drugs and the membrane potential of the smooth muscle of the guinea-pig and rat vas deferens. J Pharmacol Exp Therap 207:347–355
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Markus, R.P., Ferreira, A.T. & Lapa, A.J. Influence of castration on the membrane reactivity of the guinea-pig vas deferens. Pflugers Arch. 409, 528–532 (1987). https://doi.org/10.1007/BF00583811
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00583811