Abstract
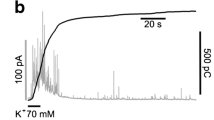
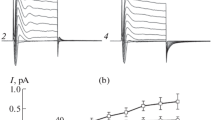
Porcine intermediate lobe (IL) endocrine cells maintained in primary culture have been studied using patch-clamp derived configurations to record unitary activity on outside-out vesicles. Solutions were devised so as to record Cl current in isolation and to fix cytoplasmic Ca concentration [Ca]i between 0.1 μM and 3 μM. Between [Ca]i 0.5 and 1 μM, the chloride permeability was restricted to single events with a small amplitude, that varied linearly with the membrane potential. Mean slope conductance of this chloride channel was 2.5 pS. Single channel analysis yielded two mean open time values of 10 and 55 ms at −80 mV. Relaxations of chloride currents on outside-out patches was examined at different [Ca]i. Relaxation was negligible at 0.15 μM [Ca]i, whereas at higher [Ca]i, the current exhibited relaxation in response to voltage jumps the kinetic of which could be fitted by two exponentials. At 0.5 μM [Ca]i, the fast relaxation time constant was shown to be voltage insensitive with a value of about 10 ms. The slow relaxation time constant had a mean value of 75 ms at −60 mV and increased with membrane depolarization with a twofold change over 120 mV. Another voltage effect was to favour the slow opening mode at the more depolarized potentials: the ratio of fast to slow relaxations being 5:1 at −60 mV as compared to 1∶1 at +80 mV). Finally the estimated probability of opening (p o) linearly increased with voltage.p o displayed a bell-shaped dependence on [Ca]i, so that full activation of the channels was not achieved.
Similar content being viewed by others
References
Bader CR, Bertrand D, Schwartz EA (1982) Voltage-activated and Ca-activated currents studied in solitary rod inner sements from the salamander retina. J Physiol (Lond) 331:253–284
Bader CR, Bertrand D, Schlichter R (1987) Calcium-activated chloride current in cultured sensory and parasympathetic quail neurones. J Physiol (Lond) 394:125–148
Barish ME (1983) A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol (Lond) 342:309–325
Boyd WH (1972) Morphological features of the hypophyseal intermediate lobe directly related to its activity. Arch Histol Jpn 34:1–17
Boyd WH, Krogsrud R (1987) Presence of α-melanocyte-stimulating hormone in bovine pituitary intraglandular colloid of intermediate lobe origin. Am J Anat 178:81–84
Demeneix BA, Taleb O, Loeffler J-PH, Feltz P (1986) GABA-A and GABA-B receptors on porcine pars intermedia cells in primary culture: functional role in modulating peptide release. Neuroscience 17:1275–1285
Evans MG, Marty A (1986) Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol (Lond) 378:437–460
Findlay I, Petersen OH (1985) Acetylcholine stimulates a Ca-dependent Cl-conductance in mouse lacrimal acinar cells. Pflügers Arch 403:328–330
Geletyuk VI, Kazachenko VN (1985) Single Cl channels in molluscan neurones: multiplicity of the conductance states. J Membr Biol 86:9–15
Gershengorn MC, Thaw C (1985) Thyrotropin-releasing hormone (TRH) stimulates biphasic elevation of cytoplasmic free calcium in GH3 cells. Further evidence that TRH mobilizes cellular and extracellular Ca. Endocrinology 116:591–596
Inoue M, Oomura Y, Yakushiji T, Akaike M (1986) Intracellular calcium ions decrease the affinity of the GABA receptor. Nature 324:156–158
Korn SJ, Weight FF (1987) Patch-clamp study of the calcium-dependent chloride current in AtT-20 pituitary cells. J Neurophysiol 58:1431–1451
Krouse ME, Schneider GT, Gage PW (1986) A large anion-selective channel has seven conductance levels. Nature 319:58–60
Leong DA, May WJ, Benedek DM, Sullivan JA, Mandell GL (1987) Increased cytosolic free calcium and ACTH release in individual corticotropes stimulated with CRF. 9th Endocrine Soc Meeting at Indianapolis, Abstr. 41
Malgaroli A, Vallar L, Elahi FR, Pozzan T, Spada A, Meldolesi J (1987) Dopamine inhibits cytosolic Ca2+ increases in rat lactotroph cells. Evidence of a dual mechanism of action. J Biol Chem 262:13920–13927
Martell EA, Smith RM (1974) Critical stability constants, vol 1. Plenum Press, New York, p 199
Marty A, Tan YP, Trautmann A (1984) Three types of calcium-dependent channel in rat lacrimal glands. J Physiol (Lond) 357:293–325
Marty A, Evans MG, Tan YP, Trautmann A (1986) Muscarinic responses in rat lacrimal glands. J Exp Biol 124:15–32
Mayer ML (1985) A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol (Lond) 364:217–239
Miledi R, Parker I (1984) Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol (Lond) 357:173–183
Neering IR, McBurney RN (1984) Role of microsomal Ca store in mammalian neurones? Nature 309:158–160
Owen DA, Segal M, Barker JL (1984) A Ca-dependent Cl conductance in cultured spinal cord neurones. Nature 311:567–570
Petersen OH, Findlay I (1987) Electrophysiology of the pancreas. Physiol Rev 67:1054–1116
Saland LC (1980) Extracellular spaces of the rat pars intermedia as outlined by lanthanum tracer. Anat Rec 196:355–361
Schlegel W, Winiger BP, Mollard P, Vacher P, Wuarin F, Zahnd GR, Wollheim CB, Dufy B (1987) Oscillation of cytosolic Ca in pituitary cells due to action potentials. Nature 329:719–721
Takahashi T, Neher E, Sakmann B (1987) Rat brain serotonin receptors in Xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc Natl Acad Sci USA 84:5063–5067.
Taleb O, Trouslard J, Demeneix BA, Feltz P, Bossu JL, Dupont JL, Feltz A (1987) Spontaneous and GABA-evoked chloride channels on pituitary intermediate lobe cells and their internal Ca requirements. Pflügers Arch 409:620–631
Taraskevich PS, Douglas WW (1985) Pharmacological and ionic features of γ-aminobutyric acid receptors influencing electrical properties of melanotrophs isolated from the rat pars intermedia. Neuroscience 14:301–308
Trouslard J, Demeneix BA, Feltz P (1988) Spontaneous spiking activities of porcine pars intermedia cells: effect of thyrotropinreleasing hormone. Neuroendocrinology (in press)
Yamamoto D, Suzuki N (1987) Blockade of chloride channels by HEPES buffer. Proc R Soc Lond [Biol] 230:93–100
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taleb, O., Feltz, P., Bossu, J.L. et al. Small-conductance chloride channels activated by calcium on cultured endocrine cells from mammalian pars intermedia. Pflugers Arch. 412, 641–646 (1988). https://doi.org/10.1007/BF00583766
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00583766