Abstract
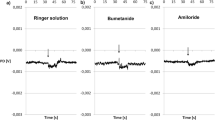
Isolated skin of the clawed frogXenopus laevis was mounted in an Ussing-chamber. The transcellular sodiumcurrent (I Na) was identified either as amiloride-blockable (10−3 mol/l) short-circuit current (I SC), or by correctingI SC for the shunt-current obtained with mucosal Tris. A dose of 10 mmol/l Cd2+ applied to the mucosal side increased the current by about 70%. The half-maximal effect was reached at a Cd2+-concentration of 2,6 mmol/l (in NaCl-Ringer). The quick and fully reversible effect of Cd2+ could not be seen when 10−3 mol/l amiloride was placed in the outer, Na+-containing solution, nor when Na+ was replaced by Tris. This suggests that Cd2+ stimulatesI Na. Cd2+ intefered with the Na+-current self-inhibition, and therefore with the saturation ofI Na by increasing the apparent Michaelis constant (K Na) of this process. The “I Na recline” after stepping up mucosal [Na+] was much reduced in presence of Cd2+. Ca2+-ions on the mucosal side had an identical effect to Cd2+, and 10 mmol/l Ca2+ increaseI Na by about 100%. The half-maximal effect was obtained with 4.4 mmol/l Ca2+. The mechanism ofI Na-stimulation by Ca2+ did not seem to differ from that of Cd2+. Thus, although of low Na+-transport capacity,Xenopus skin appears to be as good a model for Na+-transporting epithelia asRanidae skin, with the exception of the calcium effect which, so far, has not been reported forRanidae.
Similar content being viewed by others
References
Anderson J, Tomlinson RWS (1965) The effect of high and low concentrations of calcium on the sodium transport of the isolated toad bladder. J Physiol 177:133–139
Arruda JAL, Dytko G, Mola R (1979) Effect of calcium and magnesium on transport processes by the turtle bladder. Arch Int Pharmacodyn 240:27–34
Brown D, Grosso A, DeSousa RC (1981) The amphibian epidermis: distribution of mitochondria-rich cells and the effect of oxytocin. J Cell Sci 52:197–213
Chase Jr HS (1984) Does calcium couple the apical and basolateral membrane permeabilities in epithelia? Am J Physiol 247:F869-F876
Cheung WY (1984) Calmodulin: its potential role in cell proliferation and heavy metal toxicity. Fed Proc 43:2995–2999
Curran F, Gill Jr R (1962) The effect of calcium on sodium transport by frog skin. J Gen Physiol 45:625–641
Cuthbert AW, Wong PYD (1972) The role of calcium-ions in the interaction of amiloride with membrane receptors. Mol Pharmacol 8:222–229
Foulkes EC (1985) Cadmium. Springer, Heidelberg Berlin New York
Fuchs W, Hviid Larsen E, Lindemann B (1977) Current-voltage curve of Na+-channels and concentration dependence of Na+-permeability in frog skin. J Physiol (Lond) 267:137–166
Hayashi H, Takada M, Arita A (1978) Cadmium-induced decrease in the outer facing skin resistance of a bullfrog (Rana catesbeiana). Jpn J Physiol 29:63–73
Hillyard SD, Gonick HC (1976) Effect of Cd2+ on short-circuit current across epithelial membranes. J Membr Biol 26:109–119
Kaufmann AI, Erlij D (1986) K+-stimulated Na+ transport in frog skin epithelia. Pflügers Arch 407:596–601
Lansman JB, Hess P, Tsien RW (1986) Blockade of current through single calcium channels by Cd2+, Mg2+ and Ca2+. Voltage and concentration dependence of calcium-entry into the pore. J Gen Physiol 88:321–347
Lindemann B, Gebhardt U (1973) Delayed changes of Na+-permeability in response to steps of (Na+)o at the outer surface of frog skin and frog bladder. In: Ussing H, Thorns U (eds) Transport mechanisms in epithelia. Academic Press, New York, pp 115–130
Mandel LJ (1978) Effects of pH, Ca2+, ADH and theophylline on kinetics of Na+-entry in frog skin. Am J Physiol 235:C35-C48
Nagel W (1977) The dependence of the electrical potentials across the membranes of the frog skin upon the concentration of sodium in the mucosal solution. J Physiol 269:777–796
Palmer LG, Edelmann IS, Lindemann B (1980) Current-voltage analysis of apical sodium transport in toad urinary bladder: Effects of inhibitors of transport and metabolism. J Membr Biol 57:59–71
Rabito CA, Rotunno CA, Cereijido M (1978) Amiloride and calcium effect on the outer barrier of the frog skin. J Membr Biol 42:169–187
Scholtz E (1987) Elektrophysiologische Untersuchung zum Einfluß von Cadmium und Calcium auf den Na+-Transport durch die Epidermis des Krallenfrosches (Xenopus laevis). MSc Thesis, Freie Universität Berlin
Scholtz E, Zeiske W (1986) Skin of the clawed frogXenopus laevis as a model for sodium-transporting epithelia. Pflügers Arch 406:R51
Takada M, Hayashi H (1980) Effect of cadmium on active sodium transport by the abdominal skin and the isolated epidermis of the bullfrog: differences between epidermal and dermal cadmium applications. Jpn J Physiol 30:257–269
Tang J, Abramcheck FJ, Van Driessche W, Helman SI (1985) Electrophysiology and noise analysis of K+-depolarized epithelia of frog skin. Am J Physiol 249:C421-C429
Van Driessche W, Lindemann B (1979) concentration dependence of currents through single sodium-selective pores in frog skin. Nature 282:519–520
Van Driessche W, Zeiske W (1985a) Ionic channels in epithelial membranes. Physiol Rev 65:833–903
Van Driessche W, Zeiske W (1985b) Ca2+-sensitive, spontaneously fluctuating cation channels in the apical membrane of the adult frog skin epithelium. Pflügers Arch 405:250–259
Van Driessche W, Aelvoet I, Erlij D (1987) Oxytocin and cAMP stimulate monovalent cation movements through a Ca2+-sensitive, amiloride-insensitive channel in the apical membrane of the toad urinary bladder. Proc Natl Acad Sci USA 84:313–317
Yorio T, Bentley PJ (1978) The permeability of the skin of the aquatic anuranXenopus laevis (Pipidae). J Exp Biol 72:285–289
Zeiske W (1978) The stimulation of Na+-uptake in frog skin by uranyl ions. Biochim Biophys Acta 509:219–229
Zeiske W (1979) Die Na+-Aufnahme durch die apikale Membran des Froschhautepithels — ihr Mechanismus und ihre Steuerung durch Ionen und lipophile Substanzen. PhD-thesis, Universität des Saarlandes
Zeiske W (1986) Serosal K+-depolarisation alters Na+-current kinetics of frog skin in a species-dependent manner. Pflügers Arch 406:R51
Zeiske W, Lindemann B (1974) Chemical stimulation of Na+-current through the outer surface of frog skin epithelium. Biochim Biophys Acta 352:323–326
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Scholtz, E., Zeiske, W. A novel synergistic stimulation of Na+-transport across frog skin (Xenopus laevis) by external Cd2+- and Ca2+-ions. Pflugers Arch. 413, 174–180 (1988). https://doi.org/10.1007/BF00582528
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00582528