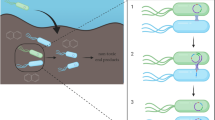
Abstract
Both the soil isolate,Pseudomonas stutzeri JM300, and the marine isolate,Pseudomonas stutzeri strain ZoBell, have been shown previously to be naturally transformable. This study reports the detection of genetic exchange by natural transformation between these two isolates. Transformation frequency was determined by filter transformation procedures. Three independent antibiotic resistance loci were used as chromosomal markers to monitor this exchange event: resistance to rifampicin, streptomycin, and nalidixic acid. The maximum frequencies of transformation were on the order of 3.1 to 3.8×10-6 transformants per recipient; frequencies over an order of magnitude greater than those for spontaneous antibiotic resistance, although they are lower than those observed for soil: soil or marine: marine strain crosses. This exchange was inhibited by DNase I. Transformation was observed between soil and marine strains, both by filter transformation using purified DNA solutions and when transforming DNA was added in the form of viable donor cells. The results from this study support the close genetic relationship betweenP. stutzeri JM300 andP. stutzeri strain ZoBell. These results also further validate the utility ofP. stutzeri as a benchmark organism for modeling gene transfer by natural transformation in both soil and marine habitats.
Similar content being viewed by others
References
Aardema BW, Lorenz MG & Krumbein WE (1983) Protection of sediment-adsorbed transforming DNA against enzymatic inactivation. Appl. Environ. Microbiol. 46: 417–420
Avery OT, MacLeod CM & McCarty M (1944) Studies on the chemical nature of the substance inducing transformation of pneumococcal types. J. Exp. Med. 79: 137–159
Bott KF & Wilson GA (1967) Development of competence inBacillus subtilis transformation system. J. Bacteriol 94: 562–570
Carlson CA & Ingraham JL (1983) Comparison of denitrification byPseudomonas stutzeri, Pseudomonas aeruginosa, andParacoccus denitrificans. Appl. Environ. Microbiol. 45: 1247–1253
Carlson CA, Pierson LS, Rosen JJ & Ingraham JL (1983)Pseudomonas stutzeri and related species undergo natural transformation. J. Bacteriol. 153: 93–99
Chauvat F, Astier C, Vedel F & Joset-Espardellier F (1983) Transformation in the cyanobacteriumSynechococcus R2: Improvement of efficiency; role of the pUH24 plasmid. Mol. Gen. Genet. 191: 39–45
Coughter JP & Stewart GJ (1989) Genetic exchange in the environment. Antonie van Leeuwenhoek 55: 15–22
Dohler K, Huss VAR & Zumft WG (1987) Transfer ofPseudomonas perfectomarina Baumann, Bowditch, Baumann, and Beaman 1983 toPseudomonas stutzeri (Lehmann and Neumann 1896) Sijderius 1946. Int. J. Syst. Bacteriol. 37: 1–3
Gallagher ML & Burke WFJr (1985) Sequence-specific endonuclease from the transformable cyanobacteriumAnacystis nidulans R2. FEMS Microbiol. Lett. 26: 317–321
Golden SS & Sherman LA (1984) Optimal conditions for genetic transformation of the cyanobacteriumAnacystis nidulans R2. J. Bacteriol. 158: 36–42
Griffith F (1928) The significance of pneumococcal types. J. Hyg. 27: 113–159
Juni E (1972) Interspecies transformation ofAcinetobacter: Genetic evidence for a ubiquitous genus. J. Bacteriol. 112: 917–931
Kahn ME & Smith HO (1984) Transformation inHaemophilus: A problem in membrane biology. J. Membr. Biol. 81: 89–103
Lee GH & Stotzky G (1989) Transformation is a mechanism of gene transfer in soil (Abstract). Wind River Conference on Genetic Exchange, Estes Park, Colorado
Levy SB & Miller RV (1989) Gene Transfer in the Environment. McGraw-Hill Publishing Company, New York
Lorenz MG, Aardema BW & Krumbein WE (1981) Interaction of marine sediment with DNA and DNA availability to nucleases. Mar. Biol. 64: 225–230
Lorenz MG & Wackernagel W (1987) Adsorption of DNA to sand and variable degradation rates of adsorbed DNA. Appl. Environ. Microbiol. 53: 2945–2952
Mandel M (1966) Deoxyribonucleic acid base composition in the genusPseudomonas. J. Gen. Microbiol. 43: 273–292
Matsubara T, Frunzke K & Zumft WG (1982) Modulation by copper of the products of nitrige respiration inPseudomonas perfectomarinus. J. Bacteriol. 149: 816–823
Palleroni NJ, Kunisawa R, Contopouolou R & Doudoroff M (1973) Nucleic acid homologies in the genusPseudomonas. Int. J. Syst. Bacteriol. 23: 333–339
Paul JH, Jeffery WH & DeFlaun MF (1987) Dynamics of extracellular DNA in the marine environment. Appl. Environ. Microbiol. 53: 170–179
Paul JH & Myers B (1982) Flurometric determination of DNA in aquatic microorganisms by use of Hoechst 33258. Appl. Environ. Microbiol. 43: 1393–1399
Smith HO, Danner DB & Deich RA (1981) Genetic transformation. Ann. Rev. Biochem. 50: 41–68
Stevens SEJr & Porter RD (1986) Heterospecific transforming among cyanobacteria. J. Bacteriol. 167: 1074–1076
Stewart GJ & Carlson CA (1986) The biology of natural transformation. Ann. Rev. Microbiol. 40: 211–235
Stewart GJ & Sinigalliano CD (1989) Detection and characterization of natural transformation in the marine bacteriumPseudomonas stutzeri strain ZoBell. Arch. Microbiol. 152: 520–526
Stewart GJ & Sinigalliano CD (1990) Detection of horizonal gene transfer by natural transformation in native and introduced species of bacteria in marine and synthetic sediments. Appl. Environ. Microbiol. 56: 1818–1824
Stewart GJ, Carlson CA & Ingraham JL (1983) Evidence for an active role of donor cells in natural transformation ofPseudomonas stutzeri. J. Bacteriol. 156: 30–35
Stotzky G (1989) Gene transfer among bacteria in soil. In: Levy SB & Miller RV (Eds) Gene Transfer in the Environment. McGraw-Hill Publishing Company, New York
Treavors JT, Barkay T & Bourquin AW (1987) Gene transfer among bacteria in soil and aquatic environments: A review. Can. J. Microbiology 33: 191–198
ZoBell CE & Upham HC (1944) A list of marine bacteria including descriptions of sixty new species. Bull. Scripps Inst. Oceanogr. Univ. Calif. 5: 239–292
Zuklic FW, Kraxberger T & Stewart GJ (1987) Detection of natural transformation in a coral surface-associated marine bacterium. 87th Annual Meeting of the American Society for Microbiology, Atlanta, GA. Abstract N-47
Zumft WG & Vega JM (1979) Reduction of nitrite to nitrous oxide by a cytoplasmic membrane fraction from the marine denitrifierPseudomonas perfectomarinus. Biochim. Biophys. Acta. 548: 484–499
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stewart, G.J., Sinigalliano, C.D. Exchange of chromosomal markers by natural transformation between the soil isolate,Pseudomonas stutzeri JM300, and the marine isolate,Pseudomonas stutzeri strain ZoBell. Antonie van Leeuwenhoek 59, 19–25 (1991). https://doi.org/10.1007/BF00582115
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00582115