Abstract
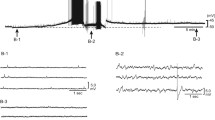
Two regulatory mechanisms on the long-term potentiation of transmitter release induced by adrenaline (adr.-l.t.p.) in bullfrog sympathetic ganglia were studied by recording intracellularly the fast excitatory postsynaptic potentials. An increase in exposure time to adrenaline from 10 min to 60 min did not enhance the magnitude of adr.-l.t.p. However, increasing an exposure time to dibutyryl cyclic AMP (1 mM) up to 60 min progressively enhanced the magnitude of the nucleotide-induced potentiation, indicating the desensitization of the β-adrenoceptor. The desensitization remained at 20 min after the removal of adrenaline in all the five cells, but disappeared at 60–90 min in four cells out of eight. Under the latter condition, the second l.tp. was summated on the first one. Dibutyryl cyclic GMP (100 μM) blocked the generation of the l.t.p. induced by dibutyryl cyclic AMP (1 mM) as well as that of adr.-l.t.p. Muscarine (10 μM) or adenosine (1 mM), a possible candidate for raising intraterminal cyclic GMP, did not significantly affect adr.-l.t.p. These results suggest that adr.-l.t.p. is regulated by the desensitization of β-adrenoceptor and a process which involves endogenous cyclic GMP acting on a step subsequent to the cyclic AMP production.
Similar content being viewed by others
References
Bliss TVP, Goddard GV, Riives M (1983) Reducing of long-term potentiation in the dentate gyrus of the rat following selective depletion of monoamines. J Physiol (Lond) 334:475–491
Goldberg ND, Haddox MK (1977) Cyclic GMP metabolism and involvement in biological regulation. Annu Rev Biochem 46:823–896
Kato E, Koketsu K, Kuba K, Kumamoto E (1985) The mechanism of the inhibitory action of adrenaline on transmitter release in bullforg sympathetic ganglia: independence of cyclic AMP and calcium ions. Br J Pharmacol 84:435–443
Koketsu K, Yamada M (1982) Presynaptic muscarinic receptors inhibiting active acetylcholine release in the bullfrog sympathetic ganglion. Br J Pharmacol 77:75–82
Kuba K, Kumamoto E (1986) Long-term potentiation of transmitter release induced by adrenaline in bull-frog sympathetic ganglia. J Physiol (Lond) 374:515–530
Kuba K, Kato E, Kumamoto E, Koketsu K, Hirai K (1981) Sustained potentiation of transmitter release by adrenaline and dibutyryl cyclic AMP in sympathetic ganglia. Nature (Lond) 291:654–656
Kumamoto E, Kuba K (1983) Independence of presynaptic bimodal actions of adrenaline in sympathetic ganglia. Brain Res 265: 344–347
Kumamoto E, Kuba K (1985) Effects of K+-channel blockers on transmitter release in bullfrog sympathetic ganglia. J Pharmacol Exp Ther 235:241–247
Kumamoto E, Kuba K (1986a) Regulatory mechanism of adrenaline-induced 1.t.p. in bullfrog sympathetic ganglia. J Physiol Soc Jpn (Abstract) 48:209
Kumamoto E, Kuba K (1986b) Mechanism of long-term potentiation of transmitter release induced by adrenaline in bullfrog sympathetic ganglia. J Gen Physiol 87:775–793
Nishi S, Koketsu K (1960) Electrical properties and activities of single sympathetic neurons in frogs. J Cell Comp Physiol 55:15–30
Ohsako S, Deguchi T (1981) Stimulation by phosphatidic acid of calcium influx and cyclic GMP synthesis in neuroblastoma cells. J Biol Chem 256:10945–10948
Palmer WK, Doukas S (1948) Dibutyryl cyclic AMP increases phosphodiesterase activity in the rat heart. Can J Physiol Pharmacol 62:1225–1230
Schwabe U, Ohga Y, Daly JW (1976) The role of calcium in the regulation of cyclic nucleotide levels in brain slices of rat and guinea pig. Naunyn-Schmiedebergs Arch Pharmacol 293:R8
Sibley DR, Lefkowitz RJ (1985) Molecular mechanisms of receptor desensitization using the β-adrenergic receptor-coupled adenylate cyclase system as a model. Nature (Lond) 317:124–129
Simpson IA, Pfeuffer T (1980) Functional desensitization of β-adrenergic receptors of avian erythrocytes by catecholamines and adenosine 3′,5′-phosphate. Eur J Biochem 111:111–116
Stanton PK, Sarvey JM (1985) The effect of high-frequency electrical stimulation and norepinephrine on cyclic AMP levels in normal versus norepinephrine-depleted rat hippocampal slices. Brain Res 358:343–348
Su Y, Harden TK, Perkins JP (1980) Catecholamine-specific desensitization of adenylate cyclase. Evidence for a multistep process. J Biol Chem 255:7410–7419
Wells JN, Hardman JG (1977) Cyclic nucleotide phosphodiesterase. Adv Cyclic Nucleotide Res 8:119–143
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kumamoto, E., Kuba, K. Mechanisms regulating the adrenaline-induced long-term potentiation in bullfrog sympathetic ganglia. Pflugers Arch. 408, 573–577 (1987). https://doi.org/10.1007/BF00581158
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00581158