Abstract
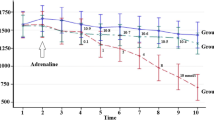
Effects of 5-hydroxytryptamine (5-HT) and forskolin on intracellular free calcium concentration ([Ca2+]i) were studied in suspensions of fura-2 loaded smooth-muscle cells from the anterior byssus retractor ‘catch’ muscle ofMytilus edulis. The successive addition of 5 mM carbachol (CCh) and 100 mM KCl to the suspension evoked a transient elevation of [Ca2+]i from the resting value of 124±2.7 nM (mean ± SE,n=18) to 300–400 nM, which was associated with contraction. The change in [Ca2+]i induced CCh was concentration-dependent with the EC50 of 10−5 M. The resting [Ca2+]i was unaffected by 10 μM 5-HT. The change in [Ca2+]i induced by 5 mM CCh was suppressed by 5-HT from 167±14.0 (n=11) to 124±14.9 (n=8) nM whereas that induced by 100 mM KCl was enhanced from 321±31.9 to 405±17.6 nM (n=8). 5-HT applied during the decaying phase of the CCh response caused a rapid decline in [Ca2+]i. In both the responses to CCh and KCl, the falling phase was accelerated by 5-HT. 10 μM forskolin, a potent activator of adenylate cyclase, mimicked the effects of 5-HT as did a membrane-permeant cyclic AMP analogue, 8-parachlorophenylthio cyclic AMP (cpt-cAMP). Application of 100 μM cpt-cAMP partially suppressed the Ca2+ i response to CCh and enhanced that to KCl.d-Tubocurarine (500 μM) added during the decaying phase of the response induced by 100 μM CCh, caused a rapid decline in [Ca2+]i similar to that caused by both 5-HT and forskolin. In essentially Ca2+-free sea water, or in the presence of 10 μM D600 in seawater containing 4 mM, Ca2+, the response to CCh was partially suppressed, whereas that to KCl was completely abolished, demonstrating a CCh-induced release of intracellularly stored Ca2+. The remaining component of the response to CCh, in either Ca2+-free sea water or in the presence of D600, was abolished by both 5-HT and forskolin. The results suggest that 5-HT has multiple effects on [Ca2+]i in the ABRM, and implicate cyclic AMP in this effect, and that one of the mechanisms underlying these responses is the inhibition of an agonist-induced release of stored Ca2+. In addition, that Ca2+ i is at, or close to resting values during the ‘catch state’.
Similar content being viewed by others
References
Achazi RK (1979) Phosphorylation of molluscan paramyosin. Pflügers Arch 379:197–201
Achazi RK, Dolling B, Haarkshorst R (1974) 5-HT-induzierte Erschlaffung und cyclisches AMP bei einem glatten Molluskenmuskel. Pflügers Arch 349:19–27
Ashley CC, Ishii N, Simpson AWM (1988) Effects of 5-hydroxytryptamine on intracellular free calcium in resting and stimulated fura-2 loaded smooth-muscle cells (ABRM) fromMytilus edulis. J Physiol (Lond) 399:21 P
Atsumi S, Sugi H (1976) Localization of calcium-accumulating structures in the anterior byssal retractor muscle ofMytulus edulis and their role in the regulation of active and catch contractions. J Physiol (Lond) 257:549–560
Bhalla RC, Webb RC, Singh D, Brock T (1978) Role of cyclic AMP in rat aortic microsomal phosphorylation and calcium uptake. Am J Physiol 234:H508-H514
Bloomquist E, Curtis B (1972) The action of serotonin on calcium-45 effluc from the anterior byssal retractor muscle ofMytilus edulis. J Gen Physiol 59:476–485
Bloomquist E, Curtis B (1975a)45Ca efflux from anterior byssus retractor muscle in phasic and catch contraction. Am J Physiol 229:1237–1243
Bloomquist E, Curtis B (1975b) Net calcium fluxes in anterior byssus retractor muscle with phasic and catch contraction. Am J Physiol 229:1244–1248
Castellani L, Cohen C (1987) Myosin rod phosphorylation and the catch state of molluscan muscles. Science 235:335–337
Cole RA, Twarog BM (1972) Relaxation of catch in a molluscan smooth muscle. I. Effects of drugs which act on the adenyl cyclase system. Comp Biochem Physiol 42A:321–330
Cooley LB, Johnson WH, Kause S (1979) Phosphorylation of paramyosin and its possible role in the catch mechanism. J Biol Chem 256:3178–3181
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Ishii N (1985) Catch contraction in single smooth muscle cells isolated from the anterior byssus retractor muscle ofMytilus edulis. J Muscle Res Cell Motil 6:374
Ishii N, Takakuwa T, Takahashi K (1986) Isolated of acetylcholine-sensitive smooth muscle cells from a molluscan catch muscle. Comp Biochem Physiol 84C:1–6
Ishii N, Simpson AWM, Ashley CC (1988) Carbachol and KCl-induced changes in intracellular free calcium concentration in isolated, fura-2 loaded smooth-muscle cells from the anterior byssus retractor muscle ofMytilus edulis. Biochem Biophys Res Commun 153:683–689
Ishii N, Simpson AWM, Ashley CC (1989) Free calcium at rest during ‘catch’ in single smooth muscle cells. Science 243:1367–1368
Itoh T, Izumi H, Kuriyama H (1982) Mechanisms of relaxation induced by activation of α-adrenoceptors in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol (Lond) 326:475–493
Jewell BR (1959) The nature of the phasic and tonic responses of the anterior byssal retractor muscle ofMytilus. J Physiol (Lond) 149:154–177
Levitan ES, Levitan IB (1988) Serotonin acting via cAMP enhances both the hyperpolarising and depolarising phases of bursting pacemaker activity in the Aplysia neuron. J Neurosci 8:1152–1161
Nauss K, Davies RE (1966) Changes in inorganic phosphate and arginine during the development, maintenance and loss of tension in the anterior byssus retractor muscle ofMytilus edulis. Biochem Z 345:173–187
Parker I, Ito Y, Kuriyama H, Miledy R (1987) α-Adrenergic agonists and cyclic AMP decrease intracellular resting free-calcium concentration in ileum smooth muscle. Proc R Soc Lond [Biol] 230:207–214
Pfil C, Plank B, Wyskovsky W, Bertel O, Hellman G, Suko J (1984) Calmodulin (Ca2+)4 is the active calmodulin-calcium species activating the calcium-, calmodulin-dependent protein kinase of cardiac sarcoplasmic reticulum in the regulation of the calcium pump. Biochim Biophys Acta 773:197–206
Poenie M, Alderton J, Tsien RY, Steinhardt RA (1985) Changes of free calcium levels with stages of cell division cycle. Nature 315:147–149
Pritchard K, Ashley CC (1986) Evidence for Na+/Ca2+ exchange in isolated smooth muscle cells: a fura-2 study. Pflügers Arch 410:401–407
Rembold CM, Murphy RA (1986) Myoplasmic calcium, myosin phosphorylation, and regulation of the crossbridge cycle in swine arterial smooth muscle. Circ Res 58:803–815
Rüegg JC (1986) Calcium in muscle activation. Springer, Berlin Heidelberg New York, p 155–164
Scanlon M, Williams DA, Fay FS (1987) A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. J Biol Chem 262:6308–6312
Saida K, van Breemen C (1984) Cyclic AMP modulation of adrenoceptor-mediated arterial smooth muscle contraction. J Gen Physiol 84:307–318
Scamon KB, Padgett W, Daly JM (1981) Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci USA 78:3663–3667
Sohma H, Yazawa M, Morita F (1985) Phosphoralation of regulatory light chain a (RLC-a) in smooth muscle myosin of scallop,Patinopecten yessoensis. J Biochem (Tokyo) 98:569–572
Sohma H, Inoue K, Morita F (1988) A cAMP-dependent regulatory protein for RLC-a myosin kinase catalysing the phosphorylation of scallop smooth muscle myosin light chain. J Biochem (Tokyo) 103:431–435
Sugi H, Yamaguchi T (1976) Activation of the contractile mechanism in the anterior byssal retractor muscle ofMytilus edulis. J Physiol (Lond) 257:549–560
Triggle DJ, Swamy VC (1983) Calcium antagonists. Some chemical-pharmacological aspects. Circ Res (Suppl I) 52:17–28
Twarog BM (1954) Responses of a molluscan smooth muscle to acetylcholine and 5-hydroxytryptamine. J Cell Comp Physiol 44:141–163
Twarog BM (1976) Aspects of smooth muscle function in molluscan catch muscle. Physiol Rev 56:829–838
Twarog BM, Muneoka Y (1973) Calcium and the control of contraction and relaxation in a molluscan catch muscle. Cold Spring Habor Symp Quant Biol 37:489–504
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ishii, N., Simpson, A.W.M. & Ashley, C.C. Effects of 5-hydroxytryptamine (serotonin) and forskolin on intracellular free calcium in isolated and fura-2 loaded smooth-muscle cells from the anterior byssur retractor (catch) muscle ofMytilus edulis . Pflugers Arch. 414, 162–170 (1989). https://doi.org/10.1007/BF00580959
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00580959