Abstract
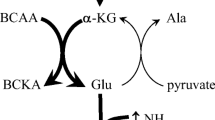
It has recently been reported that branched-chain amino acid aminotransferase (BCAATase) is inhomogeneously distributed in the kidney. BCAATase activity is several-fold higher in the medullary thick ascending limb (MTAL) than in other nephron segments. The present work was designed to determine whether leucine, a branched-chain amino acid (AA), is used as metabolic fuel by this nephron segment. MTAL were isolated from the inner stripe of the outer medulla of adult Sprague Dawley rats by mild enzymatic digestion and appropriate sieving. Leucine aminotransferase activity measured in homogenates of MTAL was 653±52 pmol α-ketoglutarate formed/μg protein per hour, a value threefold higher than that observed in the renal cortex or muscle in the same rats. Substrate oxidation was assessed by measuring14CO2 production from tracer amounts of uniformly labeled14C-amino acids or glucose in isolated MTAL incubated in modified Earle balanced salt solution. When each substrate was offered at a concentration of 1 mM, leucine oxidation was much higher than that of unbranched AA, but fivefold lower than that of glucose. With 1 mM glucose and 1 mM leucine in the medium, leucine oxidation was close to that of glucose (123±8 versus 177±15 pmol CO2/μg protein per hour), probably because glucose contributed to the formation of α-ketoglutarate, a cosubstrate for leucine transamination. Inhibition of salt transport by furosemide (0.1 mM) decreased oxidation of both substrates by 60–70%. Inhibition of salt transport by ouabain (1 mM) decreased glucose oxidation markedly. However, it doubled leucine oxidation when glucose was absent from the medium and decreased leucine oxidation by only 28% when glucose was present. This might be due to an ouabain-dependent alteration in membrane permeability to AA. These findings show that leucine is oxidized in rat MTAL and may contribute to supporting active transport in this segment. This contribution could be important after a protein meal or on high protein diet, situations in which plasma level of branched-chain AA is elevated.
Similar content being viewed by others
References
Aftring RP, Block KP, Buse MG (1986) Leucine and isoleucine activate skeletal muscle branched-chain α-keto acid dehydrogenase in vivo. Am J Physiol 250:E599-E604
Aukland K, Johannesen J, Kiil F (1969) In vivo measurements of local metabolic rate in the dog kidney. Effect of mersalyl, chlorothiazide, ethacrynic acid and furosemide. Scand J Clin Lab Invest 23:317–330
Bouby N, Trinh-Trang-Tan MM, Kriz W,Bankir L (1987) Possible role of the thick ascending limb of the urine concentrating mechanism in the protein-induced increase in GFR and kidney mass. Kidney Int 32 (suppl 22):S57-S61
Brezis M, Silva P, Epstein FH (1984) Amino acids induce renal vasodilatation in isolated perfused kidney: coupling to oxidative metabolism. Am J Physiol 247:H999-H1004
Brezis M, Rosen S, Silva P, Epstein FH (1984) Renal ischemia: a new perspective. Kidney Int 26:375–383
Burch HB, Cambon N, Lowry OH (1985) Branched-chain amino acid aminotransferase along the rabbit and rat nephron. Kidney Int 28:114–117
Chamberlin ME, Mandel LJ (1986) Substrate support of medullary thick ascending limb oxygen consumption. Am J Physiol 251:F758-F763
Chang TW, Goldberg AL (1978) Leucine inhibits oxidation of glucose and pyruvate in skeletal muscles during fasting. J Biol Chem 253:3696–3701
Cunarro JA, Weiner MW (1978) Effects of ethacrynic acid and furosemide on respiration of isolated kidney tubules: the role of ion transport and the source of metabolic energy. J Pharmacol Exp Ther 206:198–206
Dawson AG, Hird F Jr, Morton DJ(1967) Oxidation of leucine by rat liver and kidney. Arch Biochem Biophys 122:426–433
Eisenstein AB, Strack I, Gallo-Torres, H, Georgiadis A, Miller ON (1979) Increased glucagon secretion in protein-fed rats: lack of relationship to plasma amino acids. Am J Physiol 236:E20-E27
Epstein FH, Brosnan JT, Tange JD, Ross BD (1982) Improved function with amino acids in the isolated perfused kidney. Am J Physiol 243:F284-F292
Frömter E, Gessner K (1975) Effects of inhibitors and diuretics on electrical potential difference in rat proximal tubule. Pflügers Arch 357:209–224
Geigy Scientific Tables (1981) published by Ciba-Geigy Limited, Basel, Switzerland p 220
Gillim SA, Paxton R, Cook GR, Harris RA (1983) Activity state of the branched chain α-ketoacid dehydrogenase complex in heart, liver, and kidney of normal, fasted, diabetic, and protein starved rats. Biochem Biophys Res Commun 111:74–81
Goldberg DJ, Walesky M, Sherwin RS (1979) Effect of somatostatin on the plasma amino acid response to ingested protein in man. Metabolism 28:866–873
Guder WG, Ross BD (1984) Enzyme distribution along the nephron. Kidney Int 26:101–111
Guder WG, Pürschel S, Wirthensohn G (1983) Renal ketone body metabolism. Distribution of 3-oxoacid CoA-transferase and 3-hydroxybutyrate dehydrogenase along the mouse nephron. Hoppe-Seyler's Z Physiol Chem 364:S1727-S1737
Guder WG, Wagner S, Wirthensohn G (1986) Metabolic fuels along the nephron: pathways and intracellular mechanisms of interaction. Kidney Int 29:41–45
Harbhajan SP, Adibi SA (1978) Leucine oxidation in diabetes and starvation: effects of ketone bodies on branched-chain amino acid oxidation in vitro. Metabolism 27:185–200
Hintz CS, Turk WR, Cambon N, Burch HB, Nemeth PM, Lowry OH (1985) A method for branched-chain amino acid aminotransferase activity in microgram and nanogram tissue samples. Anal Biochem 146:418–422
Hus-Citharel A, Morel F (1986) Coupling of metabolic CO2 production to ion transport in isolated rat thick ascending limbs and collecting tubules. Pflügers Arch 407:421–427
Ichihara A, Koyama E (1966) Transaminase of branched chain amino acids. I. Branched chain amino acids-α-ketoglutarate transaminase. J Biochem 59:160–169
Klein KL, Wang MS, Torikai S, Davidson WD, Kurokawa K (1981) Substrate oxidation by isolated single nephron segments of the rat. Kidney Int 20:29–35
Kurokawa K, Torikai S, Wang M, Klein KL, Kawashima H (1982) Metabolic hererogeneity of the nephron. Miner Electrolyte Metab 7:225–236
Le Bouffant F, Hus-Citharel A, Morel F (1984) Metabolic CO2 production by isolated single pieces of rat distal nephron segments. Pflügers Arch 401:346–353
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mimura T, Yamada C, Swendseid ME (1968) Influence of dietary protein levels and hydrocortisone administration on the branched-chain amino acid transaminase activity in rat tissues. J Nutr 95:493–498
Mitch WE, Chesney RW (1983) Amino acid metabolism by the kidney. Miner Electrolyte Metab 9:190–202
Odessey R, Goldberg AL (1972) Oxidation of leucine by rat skeletal muscle. Am J Physiol 223:1376–1383
Seney FD Jr, Persson EG, Wright FS (1987) Modification of tubuloglomerular feedback signal by dietary protein. Am J Physiol 252:F83-F90
Trinh-Trang-Tan MM, Bouby N, Coutaud C, Bankir L (1986) Quick isolation of rat medullary thick ascending limbs. Enzymatic and metabolic characterization. Pflügers Arch 407:228–234
Wittner M, Weidtke C, Schlatter E, Di Stefano A, Greger R (1984) Substrate utilization in the isolated perfused cortical thick ascending limb of rabbit nephron. Pflügers Arch 402:52–62
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Trinh-Trang-Tan, MM., Levillain, O. & Bankir, L. Contribution of leucine to oxidative metabolism of the rat medullary thick ascending limb. Pflugers Arch. 411, 676–680 (1988). https://doi.org/10.1007/BF00580865
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00580865