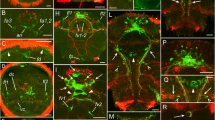
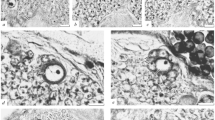
Summary
-
1.
Cellular morphogenesis during postembryonic brain development inDanaus plexippus plexippus L. was examined using histological techniques including radioautography.
-
2.
The production of new neurones is continuous throughout larval and pupal stages and shows no fluctuations corresponding to ecdysis. Glial cell production, on the other hand, occurs at the time of molting.
-
3.
New ganglion cells are formed by the division of neuroblasts found in aggregates or isolated among larval ganglion cells. Asymmetrical neuroblast divisions yield one neuroblast and one ganglion-mother cell which then divides at least once to form the new ganglion cells. Such divisions begin earlier inDanaus than in other investigated Lepidoptera. Symmetrical divisions yielding two neuroblasts also occur, but only among aggregated neuroblasts.
-
4.
Radioautographs of brains fixed at progressive intervals after Tritiated Thymidine (H3TdR) injection have permitted description of the basic pattern by which cells of the adult brain cortex are laid out and progressive changes in the relationship of new ganglion cells derived from a single neuroblast. Ganglion-mother cells are deposited between the neuroblast and the neuropile, thus forming a row of cells which move the neuroblast progressively farther from the neuropile. New ganglion cells produced by ganglion-mother cell mitoses, which usually are oriented at 45° angles to the neuropile, expand the cell cluster. Differentiating fibers of these cells are apparent within a few days of their production and seem to enter the neuropile in one bundle. Later with increased neuropile volume and further cell differentiation the cells are no longer clumped and thus are not recognizable as offspring of a single neuroblast.
-
5.
Neuroblasts found scattered among the larval ganglion cells arise from cells near the neuropile. These cells, at first indistinguishable from their neighbors, gradually assume the size and ready stainability of neuroblasts and subsequently divide according to the pattern described above.
-
6.
Scattered neuroblasts degenerate beginning shortly after pupation and have completely disappeared by the end of the fourth day.
-
7.
Except in the developing optic lobe, glial cell numbers increase through the proliferation of already existing glial cells. All glial cells show H3TdR uptake during a 12 hour period surrounding each larval-larval molt and for a somewhat longer period after pupation. However, in the larval stages mitotic figures were seen only among glial I, II, and IV. Glial I cells divide through the entire last larval stage and for two days following pupation. Large irregular mitoses seen among glial III cells at pupation indicate that these cells are probably polyploid.
-
8.
In the newly forming adult optic lobe glial II, III, and IV cells appear to develop from preganglion cells or cells indistinguishable from them. These cells gradually stain more and more darkly, segregate into the normal glial positions, and subsequently divide in accord with other glial cells.
-
9.
At the end of the fifth instar the perineurium (glial I cells), which begins to thicken during the third larval instar, is multilayered and contains many vacuolar cells. Just prior to pupation the neurilemma begins to disintegrate and during the next five days all but the cells closest to the brain disappear. Hemocytes are seen to engulf portions of the disintegrating neurilemma and already degenerating perineurial cells, but do not seem to engulf live cells. The glial I cells remaining adjacent to the brain secrete a new neurilemma.
-
10.
There is no evidence for mass destruction of larval ganglion cells by either autolysis or phagocytosis, and only in the antennal center is there evidence of degeneration of larval cells (Nordlander andEdwards, in press).
Similar content being viewed by others
References
Ashhurst, D. E., andA. G. Richards: A study of the changes occuring in the connective tissue associated with the central nervous system during the pupal stage of the wax moth. J. Morph.114, 225–256 (1964).
Bauer, V.: Zur Innern Metamorphose des Zentralnervensystems der Insecten. Zool. Jb., Abt. Anat. u. Ontog.20, 123–152 (1904).
Cajal, S. R., yD. Sanchez: Sobre la estructura de los centios opticos de los insectos. Rev. Chil. Hist. Nat. (Santiago)25, 1–18 (1921).
Glücksmann, A.: Cell deaths in normal vertebrate ontogeny. Biol. Rev.26, 59–86 (1951).
Gymer, A. L., andJ. S. Edwards: The development of the insect nervous system. I. An analysis of postembryonic growth in the terminal ganglion ofAceta domesticus. J. Morph.123, 191–197 (1968).
Hamburger, V., andR. Levi-Mantalcini: Proliferation and degeneration in the spinal ganglia of the chick embryo under normal and experimental conditions. J. exp. Zool.111, 457–502 (1949).
Hanström, B.: Comparison between the brains of the newly hatched larva and the imago ofPieris brassicae. Ent. Tidsskr.46, 43–52 (1925).
—: Vergleichende Anatomie des Nervensystems der wirbellosen Tiere. Berlin: Springer 1928.
Hertweck, H.: Anatomie und Variabilität des Nervensystems und der Sinnesorgane vonDrosophila melanogaster (Meigen). Z. wiss. Zool.139, 559–663 (1931).
Heywood, R. B.: Changes occuring in the central nervous system ofPieris brassicae L. (Lepidopteran) during metamorphosis. J. Insect. Physiol.11, 413–430 (1965).
Hinke, W.: Das relative postembryonale Wachstum der Hirnteile vonCulex pipiens, Drosophila melanogaster undDrosophila-Mutanten. Z. Morph. Ökol. Tiere50, 81–118 (1961).
Hughes, A.: Studies in embryonic and larval development in amphibia II. The spinal motor root. J. Embryol. exp. Morph.7, 128–145 (1959).
Johansen, H.: Die Entwicklung des Imagoauges vonVanessa urticae L. Zool. Jb., Abt. Anat. u. Ontog.6, 445–480 (1892).
Krishnakumaran, A., S. Berry, H. Oberlander, andH. A. Schneiderman: Nucleic acid synthesis during insect development II. Control of DNA synthesis in the Cecropia silkworm and other Saturniid moths. J. Insect. Physiol.13, 1–57 (1967).
Levi-Montalcini, R.: Growth and differentiation in the nervous system. In: The nature of biological diversity (ed.J. M. Allen), p. 261–297. New York: McGraw Hill 1963.
Lucht-Bertram, E.: Das postembryonale Wachstum von Hirnteilen beiApis mellifica L. undMyrmeleon europaeus L. Z. Morph. Ökol. Tiere50, 543–575 (1962).
Madhavan Nair, K.: Occurrence of anucleolate oocytes inChrysocaris purpureus (Westw.). Exp. Cell Res.30, 431–432 (1963).
Nordlander, R. H., andJ. S. Edwards: Morphological cell death in the postembryonic development of the insect optic lobes. Nature (Lond.)218, 780–781 (1968a).
- Morphology of the larval and adult brains of the monarch butterfly,Danaus plexippus plexippus, L. J. Morph., in press (1968b).
- Postembryonic brain development in the monarch butterfly,Danaus plexippus plexippus L. II. Development of the optic lobes. III. Development of brain centers other than the optic lobes. (In press.)
Panov, A. A.: The structure of the brain in insects in successive stages of postembryonic development. Rev. Entomol. URSS36, 269–284 (1957).
—: Structure of the insect brain at successive stages of postembryonic development. II. The central body. Rev. Entomol. URSS38, 276–284 (1959).
—: The structure of the insect brain during successive stages of postembryonic development. III. The optic lobes. Ent. Obozrenie39, 86–105 (1960a).
—: The character of reproduction of the neuroblasts, neurilemma, and neuroglial cells in the brain of the Chinese oak silkworm larvae. Dokl. Akad. Nauk SSSR132, 689–692 (1960b).
—: Ontogenetische Entwicklung des Zentralnervensystems vonAntheraea pernyi Guer. (Lepid.) Zool. Anz.167, 241–245 (1961).
—: The origin and fate of neuroblasts, neurons, and neuroglial cells in the central nervous system of the Chinese silkworm,Antheraea pernyi Guer. (Lepid., Attacidae). Rev. Entomol. URSS42, 337–350 (1963).
Pipa, R. L., andP. S. Woolever: Insect neurometamorphosis II. The fine structure of perineurial connective tissue, adipohemocytes, and the shortening ventral nerve cord of a moth,Galleria mellonella (L.) Z. Zellforsch.68, 80–101 (1965).
Power, M. E.: A quantitative study of the growth of the central nervous system of a Holometabolous insect,Drosophila melanogaster. J. Morph.91, 389–411 (1952).
Pyle, R. W.: Changes in the nervous system of Lepidoptera during metamorphosis. Thesis, Harvard University (1941).
Risler, H.: Die somatische Polyploidie in der Entwicklung der Honigbiene (Apis mellifica L.) und die Wiederherstellung der Diploidie bei den Drohnen. Z. Zellforsch.41, 1–78 (1954).
Sanchez, D.: Influence de l'histolyse des centres nerveux des insectes sur les metamorphoses. Trab. Lab. Invest. Biol. Madrid21, 385–422 (1924).
—: L'histogenèse dans les centres nerveux des insectes pendant les metamorphoses. Trab. Lab. Invest. Biol. Madrid23, 29–52 (1925).
Schrader, K.: Untersuchungen über die Normalentwicklung des Gehirns und Gehirntransplantationen bei der MehlmotteEphestia, Kühniella Zeller nebst einigen Bemerkungen über das Corpus allatum. Biol. Zbl.58, 52–90 (1938).
Sehnal, F.: Einfluß des Juvenilhormons auf Metamorphose des Oberschlundganglions beiGalleria mellonella L. Zool. Jb., Abt. allg. Zool. u. Physiol.71, 659–664 (1965).
Tiegs, O. W.: Researches on the insect metamorphosis. Roy. Soc. S. Austr.46, 222–224 (1922).
Ullman, S.: The developing nervous system inTenebrio. Phil. Trans. B252, 1–25 (1967).
Umbach, W.: Entwicklung und Bau des Komplexauges der Mehlmotte,Ephestia Kühniella Zeller, nebst einigen Bemerkungen über die Entstehung der optischen Ganglien. Z. Morph. Ökol. Tiere28, 561–594 (1934).
Viallanes, M. H.: Recherches sur l'histologie des insects et sur les phénomènes histologiques qui accompagnent le dévelopment postembryonnaire de ces animaux. Ann. Sci. nat. (Zool.)14, 1–348 (1882).
Weismann, A.: Die nachembryonale Entwicklung der Musciden nach Beobachtungen anMusca vomitoria undSarcophaga carnaria. Z. wiss. Zool.14, 187–336 (1864).
Wigglesworth, V. B.: The significance of “chromatin droplets” in the growth of insects. Quart. J. micr. Sci.83, 141–152 (1942).
—: The histology of the nervous system of an insect,Rhodnius prolixus. II. The central ganglia. Quart. J. micr. Sci.100, 299–313 (1959).
Author information
Authors and Affiliations
Additional information
This investigation was supported by training grant USPHS NIH 2 TI HD20 (RHN) and by Grant NIH NB 05137 (JSE).
Rights and permissions
About this article
Cite this article
Nordlander, R.H., Edwards, J.S. Postembryonic brain development in the monarch butterfly,Danaus plexippus plexippus, L.. W. Roux' Archiv f. Entwicklungsmechanik 162, 197–217 (1969). https://doi.org/10.1007/BF00576929
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00576929