Abstract
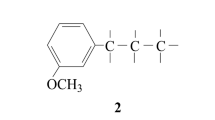
The dioxane lignin and the natural lignin of kenaf undergo 37.62% and 94.6% cleavage, respectively. The combined monomeric degradation products have been studied by the GLC method. The presence of substances relating to three types of structural units has been established: p-coumaryl, gualacyl, and syringyl. It has been shown by chromatography on Sephadex LH-20 (with ethanol-water (9:1) as solvent and eluent) that the phenolic products of degradation extracted by ethyl acetate at pH 2 consist of five fractions: oligomers, tetramers, trimers, dimers, and monomers.
Similar content being viewed by others
Literature cited
H. Nimz, Chem. Ber.,102, 799 (1969).
H. Nimz, K. Das, and N. Minemura, Chem. Ber.,104, 1871 (1971).
J. Skamla and J. Rybarik, Drev. Vys.,20, Nos. 2–3, 107 (1975).
L. S. Smirnova and Kh. A. Abdduazimov, Khim. Prir. Soedin., 505 (1978).
E. N. Yanishevskaya, B. Kh. Pulatov, and Kh. A. Abduazimov, Khim. Prir. Soedin. 737, (1980).
N. Ya. Kul'chik, G. N. Dalimova, and Kh. A. Abduazimov, Khim. Prir. Soedin., 637 (1978).
G. N. Dalimova, A. A. Geronikaki, and Kh. A. Abduazimov, Khim. Prir. Soedin., 780 (1978).
A. Yamaguchi, Mokuzai Gakkaishi,19, No. 3, 141 (1973).
Additional information
Institute of the Chemistry of Plant Substances, Academy of Sciences of the Uzbek SSR, Tashkent. Translated from Khimiya Prirodnykh Soedinenii, No. 2, pp. 234–235, March–April, 1986.
Rights and permissions
About this article
Cite this article
Dalimova, G.N., Abduazimov, K.A. Cleavage of the natural lignin and the dioxane lignin of kenaf by thioacetic acid. Chem Nat Compd 22, 218–219 (1986). https://doi.org/10.1007/BF00574745
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00574745