Summary
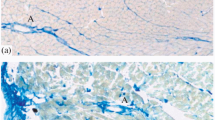
The ultrastructure of the atrial myocardium in the monkey (Macaca fascicularis) was studied after bilateral cervical vagotomy and survival times of 1, 3, 5, 7, 10, 21 and 28 days. During the first week after vagotomy, a few atrial cells showed a reduction in the sarcoplasm, crowding of the myofibrils, peripheral dispersion and reduced intercristal density of the mitochondria and increased sarcoplasmic reticulum and glycogen particles. In some profiles, there was increased electron density and granularity at the I bands and the intercalated discs. The number of such affected cells increased in the subsequent days such that by 21 to 28 days about 50% of the cells were estimated to be affected. During the latter stages further changes included, the degradation of the myofilaments and increased electron density, disorganisation and disintegration of the digital extensions at the intercalated discs. Throughout the experiments there was a leucocytic infiltration, more evident in the longer survival times.
Similar content being viewed by others
References
Ayettey AS, Navaratnam V (1978) The T-tubule system in the specialized and general myocardium of the rat. J Anat 127:125–140
Ayettey AS, Navaratnam V (1981) The ultrastructure of myocardial cells in the golden hamsterCriatus auratus. J Anat 132:519–524
Bullock GR (1986) Structural changes at cardiac cell junctions during ischaemia. J Mol Cell Cardiol 18 [Suppl 4]:1–5
Cantin M, Benchimol S, Castonguay Y, Berlinguet J-C, Huet M (1975) Ultrastructural cytochemistry of atrial muscle cells. V. Characterization of specific granules in the human left atrium. J Ultrastruct Res 52:179–192
Chernukh AM, Chernysheva GV, Kopteva LA (1974) Certain biochemical and ultrastructural features of the ventricular myocardium following cardiac denervation. Circ Res 34–35 [Suppl III]: 99–107
Chiba T, Yamauchi A (1970) On the fine structure of the nerve terminals in the human myocardium. Z Zellforsch 108:324–338
Feigl EO (1984) Parasympathetic control of coronary blood flow. Fed Proc 43:2881–2883
Forbes MS, Hawkey LA, Jirge SR, Sperkelakis N (1985) The sarcoplasmic reticulum of mouse heart: its divisions, configurations and distribution. J Ultrastruct Res 93:1–16
Forssman WG, Girardier L (1970) A study of the T system in rat heart. J Cell Biol 44:1–19
Hayat MA (1981) Fixation for electron microscopy. Academic Press, New York
Hibbs RG, Ferrans VJ (1969) An ultrastructural and histochemical study of rat atrial myocardium. Am J Anat 124:251–280
Jamieson JD, Palade GE (1964) Specific granules in atrial muscle cells. J Cell Biol 23:151–172
Korr IM, Appeltauer GSL (1974) The time course of axonal transport of neuronal proteins to muscle. Exptl Neurol 43:452–463
Korr IM, Wilkinson PN, Chornock FW (1967) Axonal delivery of neuroplasmic components to muscle cells. Science 155:342–345
Kuhn H, Richards JG, Tranzer JP (1975) The nature of rat “specific heart granules” with regard to catecholamines: an investigation by ultrastructural cytochemistry. J Ultrastruct Res 50:159–166
Löffenholz K, Pappano A (1985) The parasympathetic neuroeffector junction of the heart. Pharmacol Rev 37:1–24
MacCanon DM, Horvath SM (1957) Effect of bilateral cervical vagotomy in the dog. Am J Physiol 189:569–572
McNutt NS, Fawcett DW (1969) The ultrastructure of the cat myocardium. II. Atrial muscle. J Cell Biol 42:46–67
Metz J, Mutt V, Forssman WG (1984) Immunohistochemical localization of cardiodilatin in myoendocrine cells of the cardiac atria. Anat Embryol 170:123–127
Miledi R, Slater CR (1969) Electron-microscopic structure of denervated skeletal muscle. Proc R Soc (Lond) 174:253–269
Palmer JW, Tandler B, Hoppel CL (1977) Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252:8731–8739
Schrodt GR, Walker SM (1966) Ultrastructure of membranes in denervation atrophy. Am J Pathol 49:33–51
Tchervova IA (1960) On structural and functional alterations in heart caused by section of vagal nerves (in Russian). Arch Anat 39:13–22
Wanson J-C, Drochmans P (1972) Role of the sarcoplasmic reticulum in glycogen metabolism: binding of phosphorylase, phosphorylase kinase, and primer complexes to sarcovesicles of rabbit skeletal muscle. J Cell Biol 54:206–224
Wong WC, Ling EA, Yick TY, Tay SSW (1987) Effects of bilateral vagotomy on the ultrastructure of the cardiac ganglia in the monkey (Macaca fascicularis). J Anat 150:75–88
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wong, W.C., Yick, T.Y. & Ling, E.A. Effects of vagotomy on the ultrastructure of the atrial myocardium in the monkey (Macaca fascicularis). Anat Embryol 177, 147–152 (1987). https://doi.org/10.1007/BF00572539
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00572539