Abstract
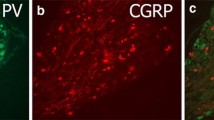
This study examined the experession of the sensory neuropeptides, calcitonin gene-related peptide (CGRP) and substance P (SP), in the lumbar 4 and 5 dorsal root ganglion (DRG) and spinal cord of spontaneously diabetic BB rats and non-diabetic controls using quantitative immunohistochemical analysis. In both animal groups immunoreactivities for CGRP and SP were widely distributed within the neurons of DRG and in nerve fibres of the dorsal spinal cord. Image analysis of each neuropeptide subpopulation in the DRG showed that in diabetic rats the cell diameter of immunostained CGRP neurons was significantly decreased compared with controls, while no difference could be found for SP-immunoreactive (IR) neurons. The decrease in the CGRP-IR cell diameter appeared to occur mainly in medium to large neurons (30–50 μm diameter; 2.2% controls, <1% diabetes), this change being parallel to an increased frequency of small-size neurons (<20 μm diameter) in diabetic rats (62% controls, 69% diabetes;P<0.05). However, there was no statistical difference in the total number of cells immunostained for either CGRP or SP between control and diabetic rats. The ratio of CGRP or SP neurons compared to total cells in the ganglion was similar in control and diabetic groups. No difference could be observed for peptide immunoreactivity in the dorsal and ventral horns of either control or diabetic animals. The observed changes of perikaryal size in diabetic rats might relate to the reduced axonal calibre and conduction velocity observed in these animals, and indicate that subpopulations of sensory neurons are affected differently by diabetes.
Similar content being viewed by others
References
Tomlinson DR Mayer JH. Defects of axonal transport in diabetes mellitus—a possible contribution to the aetiology of diabetic neuropathy. J Auton Pharmacol 4:59–72, 1984
Sidenius P, Jakobsen J. Axonal transport in human and experimental diabetes. In: Dyck PJ, Thomas PK, Asbury AK, Winergrad AI Porte D (eds) Diabetic neuropathy. Saunders, Philadelphia, London, pp 260–265, 1987
Sidenius P, Jakobsen J. Axonal transport in early experimental diabetes. Brain Res 173:315–330, 1979
Tomlinson DR, Holmes PR, Mayer JH. Reversal, by treatment with an aldose reductase inhibitor, of impaired axonal transport and motor nerve conduction velocity in experimental diabetes mellitus. Neurosci Lett 31:189–193, 1982
Tomlinson DR, Robinson JP, Willars GB, Keen P. Deficient axonal transport of substance P in streptozotocin-induced diabetic rats. Effects of sorbinil and insulin. Diabetes 37:488–493, 1988
Abate SL, Atkinson MB, Breur AC. Amount and speed of fast axonal transport in diabetes. Diabetes 40:111–117, 1991
Thomas PK, Elliasson SG. Diabetic neuropathy. In: Dyck PJ, Thomas PK, Lambert EH, Bunge R (eds) Peripheral neuropathy, Vol II. Saunders, Philadelphia, pp 1773–1810, 1984
Sima AA, Brismar T. Reversible diabetic nerve dysfunction: structural correlates to electrophysiological abnormalities. Ann Neurol 18:21–29, 1985
Sima AA, Yagihashi S. Central-peripheral distal axonopathy in the spontaneously diabetic BB-rat: ultrastructural and morphometric findings. Diabetes Res Clin Pract 1:289–298, 1985
Yagihashi S, Kamijo M, Watanabe K. Reduced myelinated fiber size correlates with loss of axonal neurofilaments in peripheral nerve of chronically streptozotocin diabetic rats. Am J Pathol 136:1365–1373, 1990
Medori R, Antilio-Gambetti L, Jenich H, Gambetti P. Changes in axon size and slow axonal transport are related in experimental diabetic neuropathy. Neurology 38:597–601, 1988
Hökfelt T, Kellerth JO, Nilsson G, Pernow B. Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res 100:235–252, 1975
Gibson SJ, Polak JM, Bloom SR, Sabate IM, Mulderry PM, Ghatei MA, McGregor GP, Morrison JFB, Kelly JS, Evans RM, Rosenfeld MG. Calcitonin gene-related peptide (CGRP) in the spinal cord of man and of eight other species. J Neurosci 4:3101–3111, 1984
Wiesenfeld-Hallin Z, Hökfelt T, Lundberg JM, Forssmann WG, Reinecke M, Tschopp FA, Fischer JA. Immunoreactive calcitonin gene-related peptide and substance P coexist in sensory neurons to the spinal cord and interact in spinal behavioral responses of the rat. Neurosci Lett 52:199–204, 1984
Ju G, Hökfelt T, Brodin E, Fahrenkrug J, Fisher JA, Frey P, Elde RP, Brown JC. Primary sensory neurons of the rat showing calcitonin gene-related peptide immunoreactivity and their relation to substance P-, somatostatin-, galanin-, vasoactive intestinal polypeptide- and cholecystokinin-immunoreactive ganglion cells. Cell Tissue Res 247:417–431, 1987
Molander C, Ygge J, Dalsgaard CJ. Substance P, somatostatin and calcitonin gene-related peptide-like immunoreactivity and fluoride resistant acid phosphatase-activity in relation to retrogradely labelled cutaneous, muscular and visceral primary sensory neurons in the rat. Neurosci Lett 74:37–42, 1987
O'Brien C, Woolf CJ, Fitzgerald M, Lindsay RM, Molander C. Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience 32:493–502, 1989
Merighi A, Polak JM, Fumagalli G, Theodosis DT. Ultrastructural localization of neuropeptides and GABA in rat dorsal horn: a comparison of different immunogold labeling techniques. J Histochem Cytochem 37:529–540, 1989
McCarthy PW, Lawson SN: Cell type and conduction velocity of rat primary sensory neurons with substance P-like immunoreactivity. Neuroscience 28:745–753, 1989
McCarthy PW, Lawson SN: Cell type and conduction velocity of rat primary sensory neurons with calcitonin gene-related peptide-like immunoreactivity. Neuroscience 34:623–632, 1990
Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol 359:31–46, 1985
Lee KH, Chung K, Chung JM, Coggeshall RE. Correlation of cell body size, axon size and signal conduction velocity for individually labelled dorsal root ganglion cells in the cat. J Comp Neurol 243:335–346, 1986
Robinson JP, Willars GB, Tomlinson DR, Keen P. Axonal transport and tissue contents of substance P in rat with long-term streptozotocin-diabetes. Effects of the aldose reductase inhibitor ‘statil’. Brain Res 426:339–348, 1987
Tomlinson DR, Willars GB, Calcutt NA, Compton AM, MacDonald IA, Clarke HE, Keen P. Effects of sorbinil treatment in rats with chronic streptozotocin-diabetes; changes in lens and in substance P and catecholamines in the iris. Curr Eye Res 8:357–363, 1989
Willars GB, Calcutt NA, Compton AM, Tomlinson DR, Keen P. Substance P levels in peripheral nerve, skin, atrial myocardium and gastrointestinal tract of rats with long-term diabetes mellitus. Effects of aldose reductase inhibition. J Neurol Sci 91:153–164, 1989
Diemel LT, Stevens EJ, Willars GB, Tomlinson DR. Depletion of substance P and calcitonin gene-related peptide in sciatic nerve of rats with experimental diabetes; effects of insulin and aldose reductase inhibition. Neurosci Lett 137:253–256, 1992
Marliss EB, Nakhooda AF, Poussier P, Sima AA. The diabetic syndrome of the ‘BB’ Wistar rat: possible relevance to type 1 (insulin-dependent) diabetes in man. Diabetologia 22:225–232, 1982
Shu S, Ju G, Fan L. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett 85:169–171, 1988
Gill J. Design and analysis of experiments in the animal and medical sciences. Iowa State University Press, vol 1, chapter 2, section 2–4, pp 185–211, 1978
Kar S, Gibson SJ, Scaravilli F, Jacobs JM, Aber VR, Polak JM. Reduced numbers of calcitonin gene-related peptide-(CGRP-) and tachykinin-immunoreactive sensory neurones associated with greater enkephalin immunoreactivity in the dorsal horn of a mutant rat with hereditary sensory neuropathy. Cell Tissue Res 255:451–466, 1989
Navarro X, Kennedy WR, Fries TJ. Small nerve fiber dysfunction in diabetic neuropathy. Muscle Nerve 12:498–507, 1989
Belai A, Burnstock G. Selective damage of intrinsic calcitonin gene-related peptide-like immunoreactive enteric fibres in streptozotocin induced diabetic rats. Gastroenterology 292:730–734, 1987
Karanth SS, Springall DR, Francavilla S, Mirrlees DJ, Polak JM. Early increase in CGRP-and VIP-immunoreactive nerves in the skin of streptozoticin-induced diabetic rats. Histochemistry 94:659–666, 1990
Lindberger M, Schröder HD, Schultzberg M, Kristensson K, Persson A, Östman J, Link H. Nerve fibre studies in skin biopsies in peripheral neuropathies. J Neurol Sci 93:289–296, 1989
Levy DM, Terenghi G, Abraham RR, Springall DR, Springall DR, Polak JM. Immunohistochemical measurements of nerves and neuropeptides in diabetic skin: relationship to tests of neurological function. Diabetologia 35:889–897, 1992
Properzi G, Francavilla S, Poccia G, Aloisi P, Gu XH, Terenghi G, Polak JM. Early increase precedes a depletion of VIP and PGP 9.5 in the skin of insulin dependent diabetics — Correlation between quantitative immunohistochemistry and clinical assessment of peripheral neuropathy. J Pathol 169:269–277, 1993
Wells MR, Vaidya U. Morphological alterations in dorsal root ganglion neurons after peripheral axon injury: association with changes in metabolism. Exp Neurol 104:32–38, 1989
Wesselmann U, Lin SF, Rymer WZ. Selective decrease of small sensory neurons in lumbar dorsal root ganglion labeled with horseradish peroxidase after ND:YAG laser irradiation of the tibial nerve in the rat. Exp Neurol 111:251–262, 1991
Medori R, Jenich H, Antilio-Gambetti L, Gambetti P. Experimental diabetic neuropathy: similar changes of slow axonal transport and axonal size in different animal models. J Neurosci 8:1814–1821, 1988
Price J. An immunohistochemical and quantitative examination of dorsal root ganglion neuronal subpopulations. J Neurosci 5:2051–2059, 1985
Tuchscherer MM, Seybold VS. Immunohistochemical studies of substance P, cholecystokinin-octapeptide and somatostatin in dorsal root ganglia of the rat. Neuroscience 14:593–605, 1985
Calcutt NA, Tomlinson DR, Willars GB, Keen P. Axonal transport of substance P-like immunoreactivity in ganglioside-treated diabetic rats. J Neurol Sci 96:283–291, 1990
Noda K, Umeda F, Ono H, Histatomi A, Chijiiwa Y, Nawata H, Ibayashi H. Decreased VIP content in peripheral nerve from streptozocin-induced diabetic rats. Diabetes 39:608–612, 1990
Belai A, Lincoln J, Milner P, Crowe R, Loesch A, Burnstock G. Enteric nerves in diabetic rats: increase in vasoactive intestinal polypeptide but not substance P. Gastroenterology 89:967–976 1985
Anand P, Llewelyn JG, Thomas PK, Gillon KRW, Lisk R, Bloom SR. Water content, vasoactive intestinal polypeptide and substance P in intact and crushed sciatic nerves of normal and streptozotocin-diabetic rats. J Neurol Sci 83:167–177, 1988
Crowe R, Burnstock G. An increase of vasoactive intestinal polypeptide-, but not neuropeptide Y-, substance P- or catecholamine-containing nerves in the iris of the streptozotocin-induced diabetic rat. Exp Eye Res 47:751–759, 1988
Di Giulio AM, Tenconi S, La Croix R, Mantegazza P, Abbracchio MP, Cattaben F, Gorio A. Denervation and hyperinnervation in the nervous system of diabetic animal. II. Monoaminergic and peptidergic alterrations in the diabetic encephalopathy. J Neurosci Res 24:362–368, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Terenghi, G., Chen, St., Carrington, A.L. et al. Changes in sensory neuropeptides in dorsal root ganglion and spinal cord of spontaneously diabetic BB rats. Acta Diabetol 31, 198–204 (1994). https://doi.org/10.1007/BF00571951
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00571951