Summary
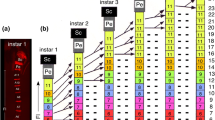
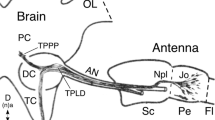
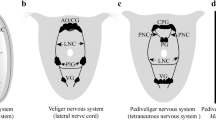
This paper describes the afferent projections of hair sensilla of the pro- and mesothoracic legs and the lateral thoracic sclerites of larval and adultTenebrio molitor and the corresponding set of pupal hair sensilla. The sensory neurons that innervate the hair sensilla of larval or adult insects project somatotopically into the thoracic neuropil. Different types of sensilla on the same region of the body surface project to the same zone of the ipsilateral thoracic ventral neuropil but exhibit different arborization patterns. Although there is a profound reorganization of body surface sensilla, the basic somatotopic layout of the larva is maintained in the adult. The sensory neurons that innervate the pupal hair sensilla possess central projections similar to those of the corresponding adult sensory neurons. The central projections of pupal sensory neurons are somatotopically oriented. Their projection pattern is serially homologous in the thoracic and the abdominal ganglia. The central projection pattern of the described pupal sensory neurons is constant throughout pupation. MAb 22C10 immunoreactivity allows an estimate of the timing of the early differentiation of the imaginal sensory neurons originating during pupation. Ablation experiments indicate that pupal sensory neurons influence the central projection pattern of the differentiating imaginal sensory neurons.
Similar content being viewed by others
References
Anderson H (1978) Postembryonic development of the visual system of the locustSchistocerca gregaria. II. An experimental analysis of the formation of the retina-lamina projection. J Embryol Exp Morphol 26:147–170
Anderson H (1985) The distribution of mechanosensory hair afferents within the locust central nervous system. Brain Res 333:97–102
Anderson H, Bacon JC (1979) Developmental determination of neuronal projection patterns from wind-sensitive hairs in the locustSchistocerca gregaria. J Embryol Exp Morphol 72:364–373
Bate CM (1973a) The mechanism of the pupal gin trap. I. Segmental gradients and the connections of the triggering sensilla. J Exp Biol 59:95–107
Bate CM (1973b) The mechanism of the pupal gin trap. II. The closure movement. J Exp Biol 59:109–119
Bate CM (1973c) The mechanism of the pupal gin trap. III. Interneurons and the origin of the closure mechanism. J Exp Biol 59:121–123
Bate CM (1978) Development of sensory systems in arthropods. In: Jacobsen M (ed) Handbook of sensory physiology, vol 9. Springer, Berlin Heidelberg New York, pp 1–53
Bräunig P, Hustert R (1980) Proprioceptors with central cell bodies in insects. Nature 283:768–770
Bräunig P, Hustert R, Pflüger HJ (1981) Distribution and specific central projections of mechanoreceptors in the thorax and proximal leg joints of locusts. I. Morphology location and innervation of internal proprioceptors of pro- and mesothorax and their central projections. Cell Tissue Res 216:57–77
Breidbach O (1987a) The fate of persisting thoracic neurons during metamorphosis of the meal beetleTenebrio molitor (Insecta: Coleoptera). Roux's Arch Dev Biol 196:93–100
Breidbach O (1987b) Constancy and variation of the serotonin-like immunoreactive neurons in the metamorphosing ventral nerve cord of the meal beetle,Tenebrio molitor L. Int J Insect Morphol Embryol 16:17–26
Breidbach O (1987c) Absence of sensory input does not affect persistent neuron inTenebrio molitor metamorphosis (Insecta: Coleoptera). Roux's Arch Dev Biol 196:486–491
Breidbach O (1987d) Einfluß des sensorischen Inputs auf die Reorganisation identifizierter Neurone in der Metamorphose des Mehlkäfers. In: Elsner N, Creutzfeldt O (eds) New frontiers in brain research. Thieme, Stuttgart
Breidbach O (1987e) Constancy of ascending projections in the metamorphosing brain of the meal-beetleTenebrio molitor L. (Insecta: Coleoptera). Wilhelm Roux's Arch 196:450–459
Breidbach O (1988a) Recorganization of identified neurons during the metamorphosis of the meal beetle — aspects for an analysis of neuronal regeneration. Monogr Dev Biol 21:105–111
Breidbach O (1988b) Die Verpuppung des Gehirns — Modell Käferhirn. Köln, Kölner Universitätsverlag
Canal I, Ferrús A (1986) The pattern of early neuronal differentiation inDrosophila melanogaster. J Neurogenet 3:293–319
Edwards JS (1969) Postembryonic development and regeneration of the insect nervous system. Adv Insect Physiol 6:97–137
Eibl E, Huber F (1979) Central projections of tibial sensory fibers within the three thoracic ganglia of cricketsGryllus campestris L.,Gryllus bimaculatus De Geer. Zoomorphology 92:1–17
Fischbach KF, Technau G (1984) Cell degeneration in the developing optic lobes of sine oculis and small-optic-lobes mutants ofDrosophila melanogaster. Dev Biol 104:219–239
Fischbach KF, Barleben F, Boschert U, Dittrich APM, Gschwander B, Houbé B, Jager R, Kaltenbach E, Ramos RGP, Schlosser G (1989) Developmental studies on the optic lobe ofDrosophila melanogaster structural brain mutants. In: Singh RN, Strausfeld NJ (eds) Neurobiology of sensory systems. Plenum, New York (in press)
Fujita SC, Zipursky SL, Benzer S, Ferrús A, Shotwell SL (1982) Monoclonal antibodies against theDrosophila nervous system. Proc Natl Acad Sci USA 79:7929–7933
Ghysen A (1978) Sensory neurons recognize defined pathways inDrosophila central nervous system. Nature 274:869–872
Ghysen A (1980) The projection of sensory neurons in the central nervous system ofDrosophila: choice of the appropriate pathway. Dev Biol 78:521–541
Hennig W (1969) Die Stammesgeschichte der Insekten. Kramer, Frankfurt
Huet C, Lenoir-Rousseaux JJ (1976) Etude de la mise en place de la platte imaginale deTenebrio molitor. I. Analyse expérimentale des processes de restauration au cours de la morphogenése. J Embryol Exp Morphol 35:303–321
Hustert R (1978) Segmental and interganglionic projections from primary fibres of insect mechanoreceptors. Cell Tissue Res 194:337–351
Hustert R, Pflüger HJ, Bräunig P (1981) Distribution and central projections of mechanoreceptors in the thorax and proximal leg joint of locust. III. The external mechanoreceptors: the campaniform sensilla. Cell Tissue Res 216:97–111
Johnson SE, Murphey RK (1985) The afferent projections of mesothoracic bristle hairs in the cricket.Acheta domesticus. J Comp Physiol [A] 156:369–379
Kent KS, Levine RB (1988) Neural control of leg movements in a metamorphic insect: persistence of larval leg motor neurons to innervate the adult legs ofManduca sexta. J Comp Neurol 276:30–43
Levine RB (1984) Changes in neuronal circuits during insect metamorphosis. J Exp Biol 112:27–44
Levine RB (1986) Reorganization of the insect nervous system during metamorphosis. Trends Neurosci 6:315–319
Levine RB, Truman JW (1983) Peptide activation of s simple neural circuit. Brain Res 279:335–338
Levine RB, Truman JW (1985) Dendritic reorganization of abdominal motoneurons during metamorphosis of the month,Manduca sexta. J Neurosci 5:2424–2431
Levine RB, Pak C, Linn D (1985) The structure, function and metamorphic reorganization of somatotopically projecting sensory neurons inManduca sexta larvae. J Comp Physil [A] 157:1–13
Murphey RK, Bacon JP, Sakaguchi DS, Johnson SE (1983) Transplantation of cricket sensory neurons to ectopic locations: arborizations and synaptic connections. J Neurosci 3:659–672
Murphey RK, Bacon JP, Johnson SE (1985) Ectopic neurons and the organization of insect sensory systems. J Comp Physiol [A] 156:381–389
Nässel DR, Geiger G (1983) Neuronal organization in fly optic lobes altered by laser ablations early in development or by mutants of the eye. J Comp Neurol 217:86–102
Obermayer M, Strausfeld NJ (1980) Silver-staining cobalt sulphide deposits within neurons of intact ganglia. In: Strausfeld NJ, Miller TA (eds) Neuroanatomical techniques. Springer, Berlin Heidelberg New York pp 44–91
Palka J (1986) Neurogenesis and axonal pathfinding in invertebrates. Trends Neurosci 482–485
Palka J, Schubinger M (1980) Formation of central patterns by receptor cell axons inDrosophila. In: Siddiqi O, Babu P, Hall L, Hall JC (eds) Development and neurobiology ofDrosophila. Plenum, New York, pp 223–246
Pflüger HJ (1980) The function of hain sensilla on the locust's leg: the role of tibial hairs. J Exp Biol 87:163–175
Pflüger HJ, Bräunig P, Hustert R (1981) Distribution and specific central projections of mechanoreceptors in the thorax and proximal leg joints of locusts. II. The external mechanoreceptors: hair plates and tactile hairs. Cell Tissue Res 216:79–96
Pflüger HJ, Bräunig P, Hustert R (1988) The organization of mechanosensory neuropiles in locust thoracic ganglia. Philos Trans R Soc Lond [Biol] 321:1–26
Pipa RL (1978) Patterns of neuronal reorganization during the postembryonic development of insects. Int Rev Cytol [Suppl] 7:403–438
Pipa RL, Cook EF, Richards AG (1959) Studies on the hexopod nervous system. II. The histology of the thoracic ganglia of the adult cockroach,Periplaneta americana (L.). J Comp Neurol 113:401–433
Poodry CA, Schneiderman A (1970) The ultrastructure of the developing leg ofDrosophila melanogaster. Wilhelm Roux's Arch 166:1–44
Snodgrass RT (1910) The thorax of insects and the articulation of the wings. Proc US Nat Mus 34 (1687):511–594
Steller H, Fischback KF, Rubin GM (1987) Disconnected: a locus required for neuronal pathway formation in the visual system ofDrosophila. Cell 50:1139–1153
Strausfeld NJ (1976) Atlas of an insect brain. Springer, Berlin Heidelberg New York
Strausfeld NJ, Singh RN (1980) Peripheral and central nervous system projections in normal and mutant (bithorax)Drosophila melanogaster. In: Siddiqi O, Babu P, Hall L, Hall JC (eds) Development and neurobiology ofDrosophila. Plenum, New York, pp 267–290
Tix S, Technau G (1987) Pioneer neurons in the optic lobes and imaginal discs ofDrosophila. In: Elsner N, Creutzfeldt O (eds) New frontiers in brain research. Thieme Verlag, Stuttgart New York, p 291
Truman JW, Levine RB (1983) Cellular events in the nervous system during metamorphosis in the mothManduca sexta. In: Current methods in cellular neurobiology. Plenum, New York
Truman JW, Reiss SE (1976) Dendritic reorganization of an identified motoneuron during metamorphosis of the tobacco hornworm moth. Science 192:477–479
Truman JW, Weeks JC, Levine RB (1985) Development plasticity during the metamorphosis of an insect nervous system. In: Cohen MJ, Sturmwasser F (eds) Comparative neurobiology. Modes of communication in the nervous system. Wiley, New York, pp 25–44
Tyrer NM, Gregory GE (1982) A guide to the neuroanatomy of locust suboesophageal and thoracic ganglia. Philos Trans R Soc Lond [Biol] 297:91–123
Tyrer NM, Bacon JP, Davies CA (1979) Sensory projections from the wind-sensitive head hairs in the locustSchistocerca gregaria. Cell Tissue Res 203:79–92
Wigglesworth VB (1957) The use of osmium in the fixation and staining of tissues. Troc R Soc Lond [Biol] 147:185–199
Wigglesworth VB (1959) The histology of the nervous system of insect,Rhodnius prolixus (Hemiptera). II. The central ganglia. Q J Microsc Sci 100:299–313
Zacharuk RY (1962) Sense organs of the head of larvae of some elateridae (Coleoptera): their distribution, structure and innervation. J Morphol 111:35–42
Zill SN, Underwood MA, Rowley JC, Moran DT (1980) A somatotopic organization of groups of afferents in insect peripheral nerves. Brain Res 198:253–269
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Breidbach, O. Metamorphic changes in the central projects of hair sensilla inTenebrio molitor L. (Insecta: Coleoptera). Cell Tissue Res. 259, 159–175 (1990). https://doi.org/10.1007/BF00571440
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00571440