Abstract
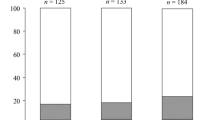
Sex determination and sex differentiation in three local Istrian (Yugoslavia) populations of the polychaeteTyposyllis prolifera were studied both in the field and the laboratory. Significant differences in sex habits were revealed among the populations, the biological significance of which is unknown. (1) Sex ratios (% ♂) in natural populations from the Adriatic Sea amounted to 50% (Rovinj), 62% (Poreč) and 77% (Pula). In the Pula-(but not in the Rovinj-)population, a correlation was found between population density on individualHalopteris scoparia thalli (the favorite habitat ofT. prolifera) and the respective sex ratio: The mean male protion was only a little more than 50% in densely populated thalli, and increased to more than 80% in sparsely populated thalli. Laboratory studies provided the information necessary to explain these field findings. (2) Laboratoryraised progenies showed an overall 1:1 primary (first sexual phase) sex ratio, independent of the local origin of the material and of whether worms were raised singly or under conditions of social contact. Nevertheless, the studied populations may differ with respect to the genetic mechanisms of sex determination: In the Poreč- and Pulapopulation individual progenies often departed significantly from the 1:1 sex ratio, whereas individual progenies in the Rovinj- population never did so. (3) The populations proved to be partially protogynous. Sex differentiation in that half of the individuals which differentiated into males (primary males) was absolutely stable during sequential sexual phases. Worms which differentiated into females, however, often changed sex at an earlier or later stage of their life cycle (secondary males). Sex change was irreversible. As to the degree of lability of female differentiation under conditions of isolation, the findings suggest a genetic divergence among both populations and individuals of the same population. Female differentiation in the Rovinj-population was virtually stable, but Pula-females underwent rapid and complete masculinization. Poreč-females were between these extremes. (4) In addition to genetic factors, exogenous conditions affected the incidence and time of sex change (Pula-population): Under conditions of social contact, sex change was delayed or suppressed as compared with isolated individuals. The degree of delay or inhibition was independent of the sex of the social partners, yet increased (to the point of saturation) with population density.
Similar content being viewed by others
Literature cited
Bacci, G.: Sex determination, 306 pp. Oxford: Pergamon Press 1965
Bacci, G.: Genetics of sex determination inOphryotrocha (Annelida Polychaeta).In: Marine organisms, pp 549–571. Ed. by B. Battaglia and J. Beardmore. New York: Plenum Press 1978
Bacci, G., M. Balata and M. L. Romani: Rapporti numerici dei sessi in tre populazioni diMytilicola intestinalis Steuer. Rend. Acc. naz. Lincei25, 557–563 (1958)
Burkenroad, M. O.: The sex ratio in alternational hermaphrodites with special reference to the determination of sexual phases in oviparous oysters. J. mar. Res.1, 75–84 (1937)
Caullery, M. and M. Comas: Le déterminisme du sexe chez un nematode (Paramermis contorta) parasite des larves de chironomes. C. r. hebd. Séanc. Acad. Sci. Paris186, 646 (1928)
Charnov, E. L. and J. Bull: When is sex environmentally determined? Nature, Lond.266, 828–830 (1977)
Coe, W. R.: Sexual phases in the American oyster (Ostrea virginica) Biol. Bull. mar. biol. Lab., Woods Hole63, 419–441 (1932)
Durchon, M.: Stolonisation et hermaphoditisme successif chezSyllis amica Quatrefage. Arch. Zool. exp. gén.88, 96–100 (1951)
Durchon, M.: Contribution à l'étude de la stolonisation chez les Syllidiens (Annélides Polychètes): I. Syllidae. Bull. biol. France Belg.93, 155–219 (1959)
Durchon, M.: Sex reversal in the Syllinae (Polychaeta: Annelida).In: Intersexuality in the animal kingdom, pp 41–47. Ed. by R. Reinboth. Berlin: Springer-Verlag 1975
Ellenby, C.: Environmental determination of the sex ratio of a plant parasitic nematode. Nature, Lond.174, 1016–1017 (1954)
Franke, H.-D.: Zur Determination der zeitlichen Verteilung von Fortpflanzungsprozessen in Laborkulturen des PolychaetenTyposyllis prolifera. Helgoländer Meeresunters.34, 61–84 (1980)
Franke, H.-D.: Endocrine mechanisms mediating light-temperature effects on male reproductive activity inTyposyllis prolifera (Polychaeta, Syllidae). Roux's Arch. Dev. Biol.192, 95–102 (1983a)
Franke, H.-D.: Endocrine control of reproductive periodicity in maleTyposyllis prolifera (Polychaeta, Syllidae). Int. J. Invertebr. Reprod.6, 229–238 (1983b)
Franke, H.-D.: On a clocklike mechanism timing lunar-rhythmic reproduction inTyposyllis prolifera (Polychaeta). J. comp. Physiol. (A)156, 553–561 (1985)
Ghiselin, M. T.: The evolution of hermaphroditism among animals. Q. Rev. Biol.44, 189–208 (1969)
Gould, H. N.: Studies on sex in the hermaphrodite molluskCrepidula plana. 3. Transference of the male producing stimules through sea water. J. exp. Zool.29, 113–120 (1919)
Gould, H. N.: Conditions affecting development of the male phase inCrepidula plana. Biol. Bull. mar. biol. Lab., Woods Hole93, 194 (1947)
Hartmann, M. and W. Huth: Untersuchungen über Geschlechtsbestimmung und Geschlechtsumwandlung beiOphryotrocha puerilis. Zool. Jahrb. allg. Zool. Physiol.56, 389–439 (1936)
Hauenschild, C.: Die phänotypische Geschlechtsbestimmung beiGrubea clavata (Annel. Polych.) und vergleichende Beobachtungen an anderen Sylliden. Zool. Jahrb. allg. Zool. Physiol.64, 15–52 (1953)
Hauenschild, C.: Weitere Kreuzungsversuche zur Frage der Geschlechtsbestimmung bei dem PolychaetenGrubea clavata. Z. Naturforsch.14b, 89–92 (1959)
Jaccarini, V., L. Agius, P. J. Schembri, and M. Rizzo: Sex determination and larval sexual interaction inBonellia viridis Rolando. (Echiura: Bonellidae). J. exp. mar. Biol. Ecol.66, 25–40 (1983)
Junqua, C.: Stolonisation et polycéphalie expérimentales chezTrypanosyllis zebra Grube (Annélide Polychète). Annls Sci. nat. Zool. Biol. animale19, 59–68 (1957)
Kegel, B. and H.-D. Pfannenstiel: Evaluation of the pair-culture effect inOphryotrocha puerilis (Polychaeta: Dorvilleidae). I. Pair-culture effect and sex ratio. Helgoländer Meeresunters.36, 205–213 (1983)
Legrand, J. J.: Contribution à l'étude expérimentale et statistique de la biologie d'Anilocra physodes. Arch. Zool. exp. gén.89, 1–55 (1952)
Leigh, E. G., E. L. Charnov and R. R. Warner: Sex ratio, sex change and natural selection. Proc. natl Acad. Sci. USA73, 3656–3660 (1976)
Leutert, R.: Sex determination inBonellia.In: Intersexuality in the animal kingdom, pp 84–90. Ed. by R. Reinboth. Berlin: Springer 1975
Parenti, U.: Ricerche sulla sessualità dei Mermitidi. 1. Rapporti sessi in una populazione diParamermis contorta. Arch. zool. ital.47, 209–224 (1962a)
Parenti, U.: Ricerche sulla sessualità dei Mermitidi. 2. Infestazione sperimentale diParamermis contorta inChironomus tentans. Boll. Zool.29, 453–462 (1962b)
Parenti, U.: Male and female influence of adult individuals on undifferentiated larvae of the parasitic nematodeParamermis contorta. Nature, Lond.207, 1105–1106 (1965)
Reinboth, R.: Can sex inversion be environmentally induced? Biol. Reprod.22, 49–59 (1980)
Reinhard E. G.: Experiments on the determination and differentiation of sex in the bobyridStegophryxus hyptius Thompson. Biol. Bull. mar. biol. Lab., Woods Hole96, 17–31 (1949)
Reverbi, G.: Ancora sulla trasformazione sperimentale del sesso nei Bopiridi. La trasformazione delle femine giovanili in maschi. Pubbl. Staz. Zool. Napoli21, 83–93 (1947)
Reverbi, G. and M. Pitotti: Il ciclo biologico e la determinazione fenotipica del sesso diIone thoracica Montagu, Bopiride parassita diCalianassa laticauda Otto. Pubbl. Staz. Zool. Napoli19, 111–184 (1942)
Schroeder, P. C. and C. O. Hermans: Annelida: Polychaeta.In: Reproduction of marine invertebrates. Vol. 3, pp 1–213. Ed. by A. C. Giese and J. S. Pearse. New York: Academic Press 1975
Warner, R. R., D. R. Robertson and E. G. Leigh: Sex change and sexual selection. Science, N.Y.190, 633–638 (1975)
Wissocq, J.-C.: Nouveaux cas d'inversion sexuelle chez les Syllidiens (Annélides Polychètes). Mém. Soc. natn Sci. nat. Math. Cherbourg51, 105–109 (1963–1964)
Author information
Authors and Affiliations
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Franke, H.D. Sex ratio and sex change in wild and laboratory populations ofTyposyllis prolifera (Polychaeta). Mar. Biol. 90, 197–208 (1986). https://doi.org/10.1007/BF00569128
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00569128