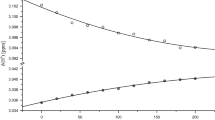
Summary
1. The UV spectra of aqueous solutions of cyclohexapeptides constructed of L(D)-alanine and glycine residues exhibit a considerable (about 45%) hypochromism of the band of the π→π* transition which has hitherto been observed among peptide-protein systems only in α-helical polypeptides.
2. In water, almost all the polypeptides studied have monotypical CD and ORD curves characterized by two Cotton effects of opposite sign in the region of the π→π* transition.
3. The features of the optical properties of the cyclohexapeptides are connected with the exciton interaction of the amide chromophores in the "pleated sheet" conformation. The neglect of such interactions may lead to high results in the determination of the degree of helicity in proteins from CD and ORD observations.
4. On passing to less polar solvents, there is a redistribution of the intensities of the Cotton effects connected with a conformational rearrangement of the cyclohexapeptides.
The authors express their gratitude to Professor E. Scoffone (Institute of Organic Chemistry, University of Padua, Italy) for kindly providing one of us (VTI) with the possibility of performing measurements on Cary-15 and JOUAN 11 instruments.
Similar content being viewed by others
Literature cited
Yu. A. Ovchinnikov, V. T. Ivanov, V. V. Shilin, and G. A. Kogan, Mol. Biol.,3, 600 (1969).
V. T. Ivanov, V. V. Shilin, Ya. Bernat, and Yu. A. Ovchinnikov, Zh. Obshch. Khim.,41, 2318 (1971).
S. L. Portnova, T. A. Balashova, V. F. Bystrov, V. V. Shilin, Ya. Bernat, V. T. Ivanov, and Yu. A. Ovchinnikov, Khim. Prirodn. Soedin.,7, 323 (1971).
V. T. Ivanov, S. L. Portnova, T. A. Balashova, V. F. Bystrov, V. V. Shilin, Yu. A. Ovchinnikov, and Ya. Bernat, Khim. Prirodn. Soedin.,7, 339 (1971).
V. T. Ivanov, L. B. Senyavina, E. S. Efremov, V. V. Shilin, and Yu. A. Ovchinnikov, Khim. Prirodn. Soedin.,7, 347 (1971).
M. Avignon, P. V. Huong, J. Lascombe, M. Marrand, and J. Neel, Biopolymers,8, 69 (1969).
H. Basch, M. B. Robin, and N. A. Kuebler, J. Chem. Phys.,47, 1201 (1967).
D. G. Barnes and W. Rhodes, J. Chem. Phys.,48, 817 (1968).
H. Basch, M. B. Robin, and N. A. Kuebler, J. Chem. Phys.,49, 5007 (1968).
W. B. Gratzer, W. Rhodes, and G. D. Fasman, Biopolymers,1, 319 (1963).
W. B. Gratzer, Poly-α-Amino Acids. Protein Models for Conformational Studies, Marcel Dekker, Inc., New York (1967), p. 182.
K. Rosenheck and P. Doty, Proc. Nat. Acad. Sci. U. S.,47, 1775 (1961).
E. B. Nielsen and J. A. Schellman, J. Phys. Chem.,71, 2297 (1967).
M. D'Alagni, B. Pipsia, and F. Quadrifoglio, La Ricerca Scientifica,38, 910 (1968).
I. Tinoco, J. Amer. Chem. Soc.,82, 4785 (1960).
W. Rhodes, J. Amer. Chem. Soc.,83, 3609 (1961).
H. De Voe, Biopolymer Symposia, No. 1, 251 (1964).
A. McLachlan and M. Ball., Mol. Phys.,8, 581 (1964).
G. Holzwarth and P. Doty, J. Amer. Chem. Soc.,87, 218 (1965).
K. Rosenheck and B. Sommer, J. Chem. Phys.,46, 532 (1967).
I. Karle and J. Karle, Acta Cryst.,16, 969 (1963).
D. W. Urry, J. Phys. Chem.,72, 3035 (1968).
D. W. Urry, A. L. Ruiter, B. C. Starcher, and T. A. Hinners, Antimicrobial Agents and Chemotherapy (1968), p. 87.
S. Laiken, M. Printz, and L. C. Craig, J. Biol. Chem.,244, 4454 (1969).
F. Quadrifoglio and D. W. Urry, J. Amer. Chem. Soc.,90, 2755 (1968).
F. Quadrifoglio and D. W. Urry, J. Amer. Chem. Soc.,90, 2760 (1968).
J. E. Shields and S. T. McDowell, J. Amer. Chem. Soc.,89, 2499 (1967).
D. Balasubramanian, J. Amer. Chem. Soc.,89, 5445 (1967).
M. A. Ruttenberg, T. P. King, and L. C. Craig, J. Amer. Chem. Soc.,87, 4196 (1965).
F. Quadrifoglio and D. W. Urry, Biochem. Biophys. Res. Commun.,29, 785 (1967).
L. C. Craig, Proc. Nat. Acad. Sci. U. S.,61, 152 (1968).
Yu. A. Ovchinnikov, V. T. Ivanov, V. F. Bystrov, A. I. Miroshnikov, E. N. Shepel, N. D. Abdullaev, E. S. Efremov, and L. B. Senyavina, Biochem. Biophys. Res. Commun.,39, 217 (1970).
K. A. Zykalova, G. N. Tishchenko, G. A. Kogan, and V. T. Ivanov, Izv. Akad. Nauk SSSR, Ser. Khim.,1970, 1547.
M. Legrand and R. Viennet, Compt. Rend.,C262, 943 (1966).
F. A. Quiocho, W. H. Bishop, and F. M. Richards, Symposium on Three-dimensional Structure of Macromolecules of Biological Origin, Proc. Nat. Acad. Sci., U. S.,57, 525 (1967).
B. W. Mattews, P. B. Sigler, R. Henderson, and D. M. Blow, Nature,241, 652 (1967).
N. Greenfield and G. D. Fasman, J. Biochem.,8, 4108 (1969).
G. M. Grippen and H. A. Scheraga, Proc. Nat. Acad. Sci. U. S.,64, 42 (1969).
D. W. Urry and P. J. Doty, J. Amer. Chem. Soc.,87, 2756 (1965).
D. D. Ulmer, J. Biochem.,4, 902 (1965).
D. E. Dickerson, M. L. Kopka, J. E. Weinzierl, J. C. Varnum, D. Eisenberg, and E. Margoliash, Abstr. 15th Natl. Meeting, Am. Chem. Soc., Chicago, Sept. (1967).
K. Blàha and I. Fric, Peptides, North-Holland Publ. Comp., Amsterdam (1968), p. 40.
K. Blàha, I. Fric, and J. Rudinger, Collection Czech. Chem. Commun.,34, 3497 (1969).
D. L. Coleman and E. R. Blout, Conformation of Biopolymers, Vol. 1, Academic Press, New York (1967), p. 123.
M. M. Shemyakin, Yu. A. Ovchinnikov, V. T. Ivanov, V. K. Antonov, E. I. Vinogradova, A. M. Shkrob, G. G. Malenkov, A. V. Evstratov, I. A. Laine, E. I. Melnik, and I. D. Ryabova, J. Membrane Biol.,1, 402 (1969).
Additional information
M. M. Shemyakin Institute of the Chemistry of Natural Compounds of the Academy of Sciences of the USSR. Translated from Khimiya Prirodnykh Soedinenii, No. 3, pp. 309–323, May–June, 1971.
Rights and permissions
About this article
Cite this article
Ivanov, V.T., Kogan, G.A., Meshcheryakova, E.A. et al. A study of the conformational states of cyclopeptide systems III. Cyclohexapeptides as systems of interacting amide chromophores. Chem Nat Compd 7, 297–306 (1971). https://doi.org/10.1007/BF00569004
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00569004