Abstract
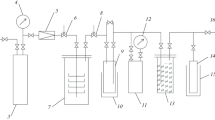
A series of aromatic compounds C6H5X (X=CH3, Cl, NO2, NH2, OCH3, CO2CH2CH3, COCH3, CN) were reacted with hydrogen in a 13.6-MHz inductively coupled glow discharge. The flow rates of aromatic and hydrogen were typically 0.5 mmol/min and 18 mmol/min, respectively. The applied power was varied from 50–200 W and the total pressure was varied from 2–14 torr. The products were collected and analyzed by gas chromatography. Three types of reactions were observed: (1) addition of hydrogen to the aromatic, (2) replacement of the group X by hydrogen, and (3) reactions characteristic of aromatic in the absence of hydrogen. The toluene reaction was studied most carefully. Methylcyclohexenes and benzene were the major products identified. The benzene was optimized by increasing the power and decreasing the pressure of either hydrogen or toluene. Reaction of toluene-d8 with hydrogen revealed that hydrogens were sequentially exchanged for deuteria on toluene and each of the products. A new apparatus is described which allows flow rates and pressure to be preselected and controlled and which allows a series of product samples to be collected without quenching the plasma.
Similar content being viewed by others
References
Y.-H. So and L. L. Miller,J. Am. Chem. Soc. 103, 4204 (1981);
N. Henis, Y.-H. So, and L. L. Miller,J. Am. Chem. Soc. 103, 4632 (1981), and references therein.
J. K. Stille, R. L. Sung, and J. Vander Kooi,J. Org. Chem. 30, 3116 (1965).
H. Suhr,Angew Chem. Int. Ed. Engl. 12, 781 (1972);Pure Appl. Chem. 39, 395 (1974); H. Suhr, inMethoden der Organische Chemie, Houben-Weyl-Müller, Edition 4, Vol. 56 (G. Thieme, Stuttgart, 1975).
International Union of Pure and Applied Chemistry, Subcommittee on Plasma Chemistry,Bibliography on Plasma Chemistry (I.U.P.A.C., 1979); available from H. Suhr, Institute for Organic Chemistry, University of Tübingen, D-74, Tübingen, West Germany.
M. Tezuka and L. L. Miller,J. Am. Chem. Soc. 100, 4201 (1978).
H. Shuhr,Z. Naturforsch. Teil B 23, 1559 (1968);
H. Suhr and U. Kunzel,Ann. Chem. 2057 (1979), and references therein.
K. Take,Bull. Chem. Soc. Jpn. 47, 1574 (1970).
M. Venugopolan, I.-S. Linn, and M. S. Grenda,J. Polym. Sci. Chem. 19, 2731 (1980).
A. Streitwieser and H. R. Ward,J. Am. Chem. Soc. 85, 539 (1963).
G. A. Brinkman,Chem. Rev. 81, 267 (1981), and references therein.
T.-S. Chen, J. Wolinska-Macydlarz, and L. C. Leitch,J. Labelled Compd. 6, 285 (1970).
H. Suhr and G. Rosskamp,Ann. Chem. 742, 43–50 (1970).
H. Suhr and R. I. Weiss,Ann. Chem. 760, 127 (1972);Z. Naturforsch. Teil B 25, 41 (1970).
M. Tokuda, L. L. Miller, A. Szabo, and H. Suhr,J. Org. Chem. 44, 4504 (1980).
G. Kruppa and H. Suhr,Ann. Chem. 677 (1980);
H. Suhr and A. Szabo,Ann. Chem. 752, 37 (1971);
H. Suhr and G. Kruppa,Ann. Chem. 744, 1 (1970).
K. Hiraoka, T. Nakamura, and K. Matsunaga,Chem. Lett. 791 (1980).
J. Gawlowski, T. Gierczak, and J. Niedzielski,J. Photochem. 13, 335 (1980).
W. A. Pryor, T. H. Lin, J. P. Stanley, and R. W. Henderson,J. Am. Chem. Soc. 95, 699 (1973).
M. F. R. Mulcahy, R. J. Harrison, and J. R. Wilmshurst,Aust. J. Chem. 19, 1431 (1966).
A. Amano, O. Horie, and N. H. Hanh,Chem. Lett. 917 (1972).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmalzl, P.W., Upham, R.A. & Miller, L.L. Reaction of hydrogen with aromatic compounds in a 13.6-MHz discharge. Plasma Chem Plasma Process 2, 43–59 (1982). https://doi.org/10.1007/BF00566857
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00566857