Abstract
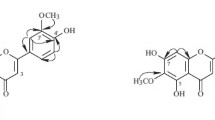
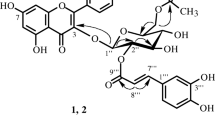
Acacetin, tilianin, linarin, and the new acylated flavone glycoside agastachoside have been isolated from the plantAgastache rugosa for the first time. On the basis of IR, UV, NMR, and mass spectra the structure of 6″-0-acetyl-7-β-D-glucopyranosyloxy-5-hydroxy-4′-methoxyflavone has been established for agastachoside.
Similar content being viewed by others
Literature cited
L. Bellamy, Infrared Spectra of Complex Molecules, 2nd ed. Wiley, New York (1958).
Z. P. Pakudina and A. S. Sadykov, The Distribution in Plants and the Physicochemical Properties of Flavones, Flavonols, and Their Glycosides [in Russian], Tashkent (1970).
T. A. Geissmann, The Chemistry of Flavonoid Compounds, Pergamon, Oxford (1962).
V. I. Litvinenko and N. P. Maksyutina, Khim. Prir. Soedin., 420 (1965).
S. Z. Ivanova, G. G. Zapesochnaya, V. I. Sheichenko, S. A. Medvedeva, and N. A. Tyukavkina, Khim. Prir. Soedin., 196 (1978).
S. Z. Ivanova, G. G. Zapesochnaya, S. A. Medvedeva, and N. A. Tyukavkina, Khim. Prir. Soedin., 200 (1978); 332 (1978).
G. G. Zapesochnaya, S. Z. Ivanova, V. I. Sheichenko, N. A. Tyukavkina, and S. A. Medvedeva, Khim. Prir. Soedin., 570 (1978).
G. G. Zapesochnaya and G. P. Shnyakina, Khim. Prir. Soedin., 806 (1978).
G. G. Zapesochnaya and G. P. Shnyakina, Khim. Prir. Soedin., 720 (1975).
L. P. Smirnova, K. I. Boryaev, and A. I. Ban'kovskii, Khim. Prir. Soedin., 96 (1974).
L. P. Smirnova, G. G. Zapesochnaya, V. I. Sheichenko, and A. I. Ban'kovskii, Khim, Prir. Soedin., 313 (1974).
T. T. Pangarova, G. G. Zapesochnaya, and E. L. Nukhimovskii, Khim. Prir. Soedin., 667 (1974).
T. T. Pangarova and G. G. Zapesochnaya, Khim. Prir. Soedin., 712 (1975).
L. S. Teslov and G. G. Zapesochnaya, Khim. Prir. Soedin., 256 (1976).
T. D. Rendyuk, A. I. Shreter, V. L. Shelyuto, and V. I. Glyzin, Khim. Prir. Soedin., 282 (1977).
G. G. Zapesochnaya, S. Z. Ivanova, S. A. Medvedeva, and N. A. Tyukavkina, Khim. Prir. Soedin., 193 (1978).
J. B. Harborne, T. J. Mabry, and H. Mabry, The Flavonoids, Chapman and Hall, London (1975), p. 400.
H. Fujiwara, G. Nonaka, A. Jagi, and J. Nishiona, Chem. Pharm. Bull.,24, No. 3, 407 (1976).
K. W. Merz and Y. H. Wu, Arch. Pharm.,274, 126 (1936).
H. Wagner, L. Hörhammer, and W. Kirchnez, Arch. Pharm.,293, 1053 (1960).
V. L. Shelyuto, V. I. Glyzin, G. N. Yurchenko, and L. P. Smirnova, Khim. Prir. Soedin., 400 (1978).
T. J. Mabry, K. R. Markham, and M. B. Thomas, The Systematic Identification of Flavonoids, Springer, New York (1970), p. 4.
Additional information
North Caucasus Zonal Experimental Station of the All-Union Scientific-Research Institute of Medical Plants, Krasnodar. Translated from Khimiya Prirodnykh Soedinenii, No. 5, pp. 642–646, September–October, 1979.
Rights and permissions
About this article
Cite this article
Zakharova, O.I., Zakharov, A.M. & Glyzin, V.I. Flavonoids ofAgastache rugosa . Chem Nat Compd 15, 561–564 (1979). https://doi.org/10.1007/BF00565924
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00565924