Abstract
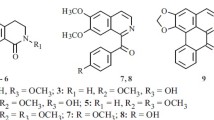
In addition to the alkaloids known previously from this plant, haplofoline and folifine, from the epigeal part ofHaplophyllum foliosum we have isolated norgraveoline with mp 288–289°C (decomp. from acetone), and myrtopsine with mp 201–202°C (from chloroform), [α]D-5° (c 0.05; methanol), which were first obtained fromHaplophyllum dubium andMyrtopsis sellingii, respectively. We have confirmed by the PMR-spectroscopic method the positions of the substituents in the dihydrofuran ring of myrtopsine suggested previously on the basis of biogenetic considerations. In addition, it has been established that the substituents are present in the trans form. This is the first time that myrtopsine has been detected in plants of the genusHaplophyllum.
Similar content being viewed by others
Literature cited
V. I. Akhmedzhanova, I. A. Bessonova, and S. Yu. Yunusov, Khim. Prirodn. Soedin., 803 (1980).
D. M. Razakova, I. A. Bessonova, and S. Yu. Yunusov, Khim. Prirodn. Soedin., 810 (1979).
I. A. Bessonova and S. Yu. Yunusov, Khim. Prirodn. Soedin., 303 (1977).
M. S. Hifnawy, J. Vaquette, T. Sevenet, J. L. Pousset, and A. Cave, Planta Med.,29, 346 (1976).
M. F. Grundon, D. M. Harrison, and C. G. Spyropoulos, J. Chem. Soc. Chem. Commun., 51 (1974).
A. V. Robertson, Aust. J. Chem.,16, 451 (1963); K. L. Seitanidi and M. R. Yagudaev, Khim. Prirodn. Soedin., 755 (1974); I. A. Bessonova, Ya. V. Rashkes, and S. Yu. Yunusov, Khim. Prirodn. Soedin., 358 (1974).
N. S. Bhacca and D. Williams, Applications of NMR Spectroscopy in Organic Chemistry, Holden-Day, San Francisco (1964).
S. R. Johns and J. A. Lamberton, Aust. J. Chem.,19, 1991 (1966).
Sh. Faizutdinova, I. A. Bessonova, and S. Yu. Yunusov, Khimiya Prirodn. Soedin., 257 (1967); I. M. Fakhrutdinova, G. P. Sidyakin, and S. Yu. Yunusov, Uzb. Khim. Zh., No. 4, 41 (1963).
D. M. Razakova, I. A. Bessonova, and S. Yu. Yunusov, Khim. Prirodn. Soedin., 682 (1976).
Additional information
Institute of the Chemistry of Plant Substances of the Academy of Sciences of the Uzbek SSR, Tashkent. Translated from Khimiya Prirodnykh Soedinenii, No. 5, pp. 613–616, September–October, 1981.
Rights and permissions
About this article
Cite this article
Akhmedzhanova, V.I., Bessonova, I.A. Alkaloids ofHaplophyllum foliosum. II.. Chem Nat Compd 17, 447–449 (1981). https://doi.org/10.1007/BF00565160
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00565160