Summary
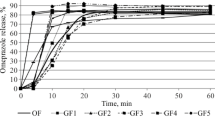
The absorption of quinidine from single and multiple doses of an enteric-coated preparation (Systodin®) was studied in seven healthy subjects, and was compared with the pharmacokinetics of intravenously administered quinidine and the results of in vitro dissolution tests of the tablets. Absorption of quinidine began after a variable delay, 2–8 h (mean 4.8) after fasting and 3–10 h (mean 6.1) after food. The rate of absorption varied both in and between individuals. It appeared to be lower when the drug was administered after food. Multiple doses after food gave a pattern of plasma concentration-time curves similar to that found on administration of single doses after food. The delay prior to absorption was prolonged at night. The ratio between the maximum and minimum concentration of quinidine during a dose interval varied from 1.3 to 3.2 (mean 2.0). Bioavailability of quinidine in fasting subjects ranged from 69 to 95% (mean 83); variation was greater when doses were administered after food. The release of quinidine from the enteric-coated preparation was pH dependent and was sustained at low pHs as may be found in the intestines. The results indicate that the absorption of quinidine from the enteric-coated formulation was dependent on the highly variable rate of gastric emptying and the pH of intestinal fluid, and it varied greatly both within and between individuals.
Similar content being viewed by others
References
Bolme, P., Otto, U.: Dose-dependence of the pharmacokinetics of quinidine. Eur. J. clin. Pharmacol.12, 73 (1977)
Conrad, K.A., Molk, B.L., Chidsey, C.A.: Pharmacokinetics of quinidine in patients with arrhythmias. Circulation55, 1 (1977)
Cramér, G., Isaksson, B.: Quantitative determination of quinidine in plasma. Scand. J. Clin. Lab. Invest.15, 553 (1963)
Ditlefsen, E.M.L.: Concentration of quinidine in blood following oral, parenteral and rectal administration. Acta Med. Scand.146, 49 (1954)
Ditlefsen, E.M.L., Løken, H.F.: Quinidine concentrations in serum following two different types of delayed-absorption tablets. Acta Med. Scand.179, 1 (1966)
Ditlefsen, E.M.L., Bjerkelund, C.: Quinidine concentration in serum. The stability during maintained treatment with two different types of delayed absorption tablets. Acta Med. Scand.180, 537 (1966)
Fordtran, J.S., Locklear, T.W.: Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am. J. Dig. Dis.11, 503 (1966)
Frigo, G.M., Perucca, E., Teggia-Droghi, M., Gatti, G., Mussini, A., Salerno, J.: Comparison of quinidine plasma concentration curves following oral administration of some short- and long-acting formulations. Br. J. Clin. Pharmacol.4, 449 (1977)
Gibaldi, M., Perrier, D.: Pharmacokinetics. New York: Marcel Dekker 1975
Greenblatt, D.J., Pfeifer, H.J., Ochs, H.R., Franke, K., MacLaughlin, D.S., Smith, T.W., Koch-Weser, J.: Pharmacokinetics of quinidine in humans after intravenous, intramuscular and oral administration. J. Pharmacol. Exp. Ther.202, 365 (1977)
Härtel, G., Louhija, A., Konttinen, A., Halonen, P.I.: Value of quinidine in maintenance of sinus rhythm after electric conversion of atrial fibrillation. Br. Heart. J.32, 57 (1970)
Mahon, W.A., Mayersohn, M., Inaba, T.: Disposition kinetics of two oral forms of quinidine. Clin. Pharmacol. Ther.19, 566 (1976)
Levy, G., Hollister, L.E.: Failure of U.S.P. Disintegration test to assess physiological availability of enteric coated tablets. N.Y. State J. Med.64, 3002 (1964)
Mason, W.D., Covinsky, J.O., Valentine, J.L., Kelly, K.L., Weddle, O., Martz, B.L.: Comparative plasma concentrations of quinidine following administration of one intramuscular and three oral formulations to 13 human subjects. J. Pharm. Sci.65, 1325 (1976)
Nimmo, W.S.: Drugs, diseases and altered gastric emptying. Clin. Pharmacokin.1, 189 (1975)
Resnekov, L., Gibson, D., Waich, S., Muir, J., McDonald, L.: Sustained-release quinidine (Kinidin Durules) in maintaining sinus rhythm after electroconversion of atrial dysrhythmias. Br. Heart. J.33, 220 (1971)
Sampson, J.J., Foreman, H., Solomon, B.C.: Studies of plasma quinidine content III. The value of delayed absorption coated tablets in oral quinidine therapy. Circulation5, 534 (1952)
Sokolow, M., Ball, R.E.: Factors influencing conversion of chronic atrial fibrillation with special reference to serum quinidine concentration. Circulation14, 568 (1956)
Strum, J.D.: Colaizzi, J.L., Jaffe, J.M., Martineau, P.C., Poust, R.I.: Comparative bioavailability of four commercial quinidine sulfate tablets. J. Pharm. Sci.66, 539 (1977)
Ueda, C.T., Hirschfeld, D.S., Scheinman, M.M., Rowland, M., Williamson, B.J., Dzindzio, B.S.: Disposition kinetics of quinidine. Clin. Pharmacol. Ther.19, 30 (1976)
Ueda, C.T., Williamson, B.J., Dzindzio, B.S.: Absolute quinidine bioavailability. Clin. Pharmacol. Ther.20, 260 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fremstad, D., Nilsen, O.G., Amlie, J. et al. Absorption of quinidine from an enteric-coated preparation. Eur J Clin Pharmacol 16, 107–112 (1979). https://doi.org/10.1007/BF00563116
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00563116