Summary
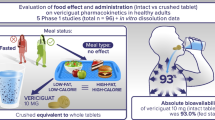
In eight healthy volunteers the bioavailability of β-acetyldigoxin solution and tablets was measured after single and multiple doses. Plasma and urine data were used to calculate bioavailability by four different methods. The differences obtained and the interindividual variations suggested that different and independent methods should be employed to assess “absolute” and “true” bioavailability. There was no significant difference between the regimens and both preparations had similar bioavailability; mean values (± SD) for the solution were 68.3 ± 8.7 % (single dose) and 68.6±5.9 % (multiple doses), and for the tablets 72.8±7.5 % and 66.1±6.0 %, respectively. For routine measurements, single dose studies with plasma and urine sampling for 24 hours and 48 hours, respectively, were an accurate and practicable procedure.
Similar content being viewed by others
References
Berman, M., Weiss, M.F.: SAAM-Manual, Laboratory of Theoretical Biology. Bethesda, Maryland: NIH 1974
Binnion, P.F.: The absorption of digoxin tablets. Clin. Pharmacol. Ther.16, 807–812 (1974)
Fraser, E.J., Leach, R.H., Poston, J.W., Bold, A.M., Culank, L.S., Lipede, A.B.: Dissolution and bioavailability of digoxin tablets. J. Pharm. Pharmacol.25, 968–973 (1973)
Greenblatt, D.M., Duhme, D.W., Koch-Weser, J., Smith, R.W.: Intravenous digoxin as a bioavailability standard: Slow infusion and rapid injection. Clin. Pharmacol. Ther.15, 510–513 (1974)
Greenblatt, D.J., Duhme, D.W., Koch-Weser, J., Smith, T.W.: Equivalent bioavailability from digoxin elixir and rapid-dissolution tablets. J. Amer. med. Ass.229, 1774–1776 (1974a)
Greenblatt, D.J., Duhme, D.W., Koch-Weser, J., Smith, T.W.: Comparison of one- and six-day urinary digoxin excretion in single-dose bioavailability studies. Clin. Pharmacol. Ther.16, 813–816 (1974b)
Greenblatt, D.J., Smith, T.W., Koch-Weser, J.: Bioavailability of drugs: The digoxin dilemma. Clin. Pharmacokin.1, 36–51 (1976)
Heizer, W.D., Smith, T.W., Goldfinger, S.E.: Absorption of digoxin in patients with malabsorption syndromes. New Engl. J. Med.285, 257–259 (1971)
Huffman, D.H., Azarnoff, D.L.: Absorption of orally given digoxin preparations. J. Amer. med. Ass.222, 957–960 (1972)
Huffman, D.H., Manion, C.V., Azarnoff, D.L.: Absorption of digoxin from different oral preparations in normal subjects during steady state. Clin. Pharmacol. Ther.16, 310–317 (1974)
Klink, P.R., Poust, R.I., Colaizzi, J.L., McDonald, Jr., R.H.: Bioavailability and dissolution properties of two commercial digoxin tablets. J. Pharm. Sci.63, 1231–1234 (1974)
Kramer, W.G., Lewis, R.P., Cobb, T.C., Forester, Jr., W.F., Visconti, J.A., Wanke, L.A., Boxenbaum, H.G., Reuning, R.H.: Pharmacokinetics of digoxin: Comparison of a two- and three-compartment model in man. J. Pharmacokin. Biopharm.2, 299–312 (1974)
Lindenbaum, J., Mellow, M.H., Blackstone, M.O., Butler, Jr., V.P.: Variation in biologic availability of digoxin from four preparations. New Engl. J. Med.285, 1344–1347 (1971)
Lindenbaum, J.: Bioavailability of digoxin tablets. Pharmacol. Rev.25, 229–237 (1973)
Lindenbaum, J., Preibisz, J.J., Butler, Jr., V.P., Saha, J.R.: Variation in digoxin bioavailability: A continuing problem. J. chron. Dis.26, 749–754 (1973)
Nyberg, L., Bratt, L., Forsgren, A., Hugosson, S.: Bioavailability of digoxin from tablets. Acta pharm. Suecica11, 447–458 (1974)
Ochs, H., Bodem, G., Hahn, E., Dengler, J.J.: Vergleichende Untersuchungen der biologischen Verfügbarkeit von Digoxin aus Tabletten und Lösung im Langzeitversuch. Klin. Wschr.53, 425–429 (1975)
Preibisz, J.J., Butler, Jr., V.P., Lindenbaum, J.: Digoxin tablet bioavailability: Single-dose and steady-state assessment. Ann. intern. Med.81, 469–474 (1974)
Rietbrock, M., Guggenmos, J., Kuhlmann, J., Hess, U.: Bioavailability and pharmacokinetics of β-methyldigoxin after multiple oral and intravenous doses. Europ. J. clin. Pharmacol.9, 373–379 (1976)
Shah, N., Pytelewski, R., Eisen, J., Jarowski, C.I.: Influence of dispersion method on dissolution rate and bioavailability of digoxin from triturations and compressed tablets. J. pharm. Sci.63, 339–343 (1974)
Shaw, T.R.D., Carless, J.E.: The effect of particle size on the absorption of digoxin. Europ. J. clin. Pharmacol.7, 269–273 (1974)
Smith, T.W., Butler, J.V.P., Haber, E.: Determination of therapeutic and toxic serum concentrations by radioimmunoassay. New Engl. J. Med.281, 1212–1216 (1969)
Steinness, E., Christensen, V., Johansen, H.: Bioavailability of digoxin tablets. Clin. Pharmacol. Ther.14, 949–954 (1973)
Wagner, J.G., Christensen, M., Sakmar, E., Blair, D., Yates, J.D., Willis III, P.W., Sedman, A.J., Stoll, R.G.: Equivalence lack in digoxin plasma levels. J. Amer. med. Ass.224, 199–204 (1973)
White, R.J., Chamberlain, D.A., Howard, M., Smith, T.W.: Plasma concentration of digoxin after oral administration in the fasting and postprandial state. Brit. med. J.1971 I, 380–381
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Klotz, U., Antonin, K.H. & Bieck, P.R. Bioavailability of β-acetyldigoxin after single and repeated doses of tablets and solution. Eur J Clin Pharmacol 10, 417–424 (1976). https://doi.org/10.1007/BF00563078
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00563078