Summary
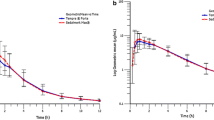
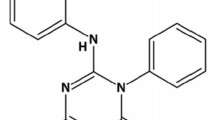
The bioavailability of orally administered hydralazine was assessed in 4 healthy subjects after separate administration of a single oral or intravenous dose (0.3 mg·kg−1). Comparison of the areas under the serum concentration-time curves showed that 26 – 55 % of the oral dose was available to the systemic circulation as unchanged drug. The O - 24 h excretion of the drug in urine was rapid: 11.4 – 14.1 % of the dose after intravenous administration, and 2.0 – 3.6 % after an oral dose. Acetylation of hydralazine leads to formation of 3-methyl-s-triazolo-3,4,a-phthalazine (MTP) and a gas-liquid-chromatographic method for its measurement in urine was developed. After oral and intravenous administration, 0.8 – 1.2 % and 1.4 – 2.3 % of the dose, respectively, were recovered within 24 hours from urine as MTP. After oral administration there was a relative increase in the amount of MTP in every subject, which indicates route-dependent metabolism. The lower bioavailability of oral hydralazine could be explained in terms of first-pass metabolism.
Similar content being viewed by others
References
Israili, Z.H., Dayton, P.G., Segal, J.L., Leifer, C.E., O'Malley, K., Gilbert, J.C., McNay, J.L.: Further studies with hydralazine-14C (H-14C) and metabolites. Abstract from paper presented at The VIth International Congress of Pharmacology, Helsinki, July 20 – 25th, 1975; p. 579
Lesser, J.M., Israili, Z.H., Davis, D.C., Dayton, P.G.: Metabolism and disposition of hydralazine-14C in man and dog. Drug. Metab. Disposit.2, 351–360 (1974)
Zak, S.B., Bartlett, M.F., Wagner, W.E., Gilleran, T.G., Lukas, G.: Disposition of hydralazine in man and a specific method for its determination in biological fluids. J. Pharm. Sci.2, 225–229 (1974)
Wagner, J.: Pharmacokinetics and metabolism of hydralazine: Specific affinity for blood vessels. Experientia (Basel)29, 767 (1973)
Evans, D.A.P., White, T.: Human acetylation polymorphism. J. Lab. clin. Invest.63, 395–405 (1964)
Edwards, S., Marquardt, F.-H.: The metabolism of 1-hydrazinophthalazine. The correct structure of the pseudo-“N-acetyl-1-hydrazinophthalazine”. Hoppe-Seylers Z. physiol. Chem.350, 85–86 (1969)
Zimmer, H., Kokosa, J., Garteiz, D.A.: Identification of 3-methyl-s-triazolo-3,4,a-phthalazine, a human hydralazine metabolite, by gas chromatography — mass spectrometry. Arzneimittel-Forsch.23, 1028–1029 (1973)
Talseth, T.: Studies on hydralazine. I. Serum concentrations of hydralazine in man after single dose and at steady-state. Europ. J. clin. Pharmacol.10, 183–187 (1976)
Zacest, R., Koch-Weser, J.: Relation of hydralazine plasma concentrations to dosage and hypotensive action. Clin. Pharmacol. Ther.13, 420–425 (1972)
Zak, S.B., Gilleran, T.G., Karliner, J., Lukas, G.: Identification of two new metabolites of hydralazine from human urine. J. med. Chem.17, 381–382 (1975)
Zimmer, H., Glaser, R., Kokosa, J.: 3-Hydroxymethyl-s-triazolo-3,4,a-phthalazine, a novel urinary hydralazine metabolite in man. J. med. Chem.18, 1031–1033 (1975)
Jack, D.B., Brechbühler, S., Degen, P.H., Zbinden, P., Riess, W.: The determination of hydralazine in plasma by gas-liquid-chromatography. J. Chromatogr.115, 87–92 (1975)
Dost, F.H.: Grundlagen der Pharmakokinetik. 2. Auflage, pp. 391–397. Stuttgart: Georg Thieme Verlag 1968
Greenblatt, D.J., Koch-Weser, J.: Clinical pharmacokinetics. New Engl. J. Med.293, 702–705 (1975)
Mayersohn, M., Gibaldi, M.: Mathematical methods in pharmacokinetics. II. Solution of the two compartment open model. Amer. J. pharm. Educ.35, 19–28 (1971)
Jenne, J.W.: Isoniazid acetylation by human liver and intestinal mucosa. Fed. Proc.22, 540–545 (1973)
Hearse, D.J., Weber, W.W.: Multiple N-acetyl-transferases and drug metabolism. Tissue distribution, characterization and significance of mammalian N-acetyltransferases. Biochem. J.132, 519–526 (1973)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Talseth, T. Studies on hydralazine. Eur J Clin Pharmacol 10, 395–401 (1976). https://doi.org/10.1007/BF00563075
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00563075