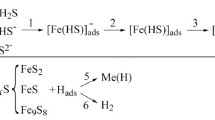
Abstract
We study the influence of preliminary electrolysis of the hydrogenation solution on the absorption of hydrogen by 65G steel. We establish that, in order for a hydrogenation solution to reach a stable active state, it is necessary that at least 0.1 A·h/liter of electricity pass through this solution. For activated and fresh solutions, the diffusion coefficients are found from plots of logi e-t. By integrating the functionsi e=f(t), we determine the amount of hydrogen that was extracted from steel for various hydrogenation times. The influence of nickel and palladium protective layers on the absorption of hydrogen by 65G steel is examined. It is discovered that the palladium protective layers facilitate substantial hydrogenation of the base metal.
Similar content being viewed by others
References
S. M. Beloglazov, “Effect of colloidal selenium, tellurium, phosphorus and vanadium pentoxide on the hydrogenation of steel cathodes,”Uch. Zap. Permskogo Gos. Univ. 19. No. 1, 33–36 (1961).
M. N. Polukarov and N. A. Apollov, “Effect of selenium compounds on the saturation of steel by electrolytic hydrogen and changes in its elastic properties,”Zh. Prikl. Khim., No. 10, 237–244 (1937).
M. N. Polukarov, “Ultramicroscopic investigation of the electrolysis of solutions of sulfurous and selenic acids and their mixtures with sulfuric acid,”Uch. Zap. Permskogo Gos. Univ. 8, 115–123 (1953).
S. S. Chatterjee, B. G. Ateya, and H. W. Pickering, “Effect of electrodeposited metals on the permeation of hydrogen through iron membranes,”Metall. Trans. A,9, No. 3, 389–395 (1978).
R. Driver, “Electrodeposition of palladium on iron and steel,”Electochem. Soc. 128, No. 11, 2367–2369 (1981).
M. Iino, “Trapping of hydrogen by sulfur-associated defects in steel,”Metall. Trans. A. 16A, No. 3, 401–410 (1985).
M. Yamashita and H. Takemura, “Electrocatalytic activity and corrosion behavior of nickel and/or palladium deposited on α-iron membrane for fuel cell anodes,”37th ISE Meeting, Vilnius, Vol. 4 (1968), pp. 264–266.
N. G. Krapivnyi, “Use of electrochemical extraction to the study for hydrogenation of metals,”Elektrokhimiya 17, No. 9, 1174–1178 (1982).
Additional information
Dnepropetrovsk Institute of Chemical Engineering, Dnepropetrovsk. Translated from Fiziko-Khimicheskaya Mekhanika Materialov, Vol. 29, No. 6, pp. 22–26, November–December, 1993.
Rights and permissions
About this article
Cite this article
Sobornitskii, V.I., Kleshnya, V.B. & Krapivnyi, N.G. Kinetics of hydrogen absorption by steel with protective coatings on the surface. Mater Sci 29, 575–579 (1994). https://doi.org/10.1007/BF00561631
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00561631