Summary
The nonlinear disposition kinetics of 5-fluorouracil (5-FU) were investigated in 6 patients with colorectal carcinoma. Each patient randomly received two single, intravenous doses of 5-FU (7.5 and 15 mg/kg) on separate days. Venous blood and urine samples were collected just prior to and for 5 h after drug administration. In addition to the kinetic studies, the in vitro whole blood/plasma concentration ratio and stability of 5-FU at 37°C were determined in whole blood from normal volunteers and from 5 patients with colorectal carcinoma.
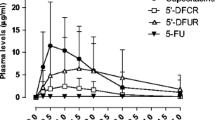
A disproportionate increase in area under the curve and corresponding decrease in total body clearance with increasing dose was observed suggesting dose-dependent behavior of 5-FU. Doubling the dose was accompanied by a 36% decrease in nonrenal clearance but no apparent change in renal clearance. Therefore, the mechanism for dose-dependent elimination appears to be primarily associated with nonrenal processes. The mean 5-FU half-life following the high dose was nearly twice as long as that observed for the low dose (12.3 versus 6.2 min). The log-linear decline in plasma concentrations and increase in half-life with dose suggest the potential role of product-inhibition as an explanation for the observed nonlinearity in 5-FU elimination.
The present study demonstrates that 5-FU degrades when incubated in whole blood. This most likely reflects metabolism in red blood cells or other blood-formed elements since 5-FU was stable in plasma. Although degradation in whole blood occurs, the estimated whole blood clearance does not contribute significantly to the observed total body clearance value. These findings suggest the possibility of pulmonary clearance of 5-FU.
Similar content being viewed by others
References
Myers C (1981) The pharmacology of the fluropyrimidines. Pharmacol Rev 33: 1–15
Gustavsson B, Hafstrom L (1981) Adjuvant and pallative treatment of colorectal cancer with fluorinated pyrimidines. A pharmacological and clinical review. Acta Chir Scand [Suppl] 504: 1–28
Collins J, Dedrick R, King F, Speyer J, Myers C (1980) Nonlinear pharmacokinetic models for 5-fluorouracil in man: Intravenous and intraperitoneal routes. Clin Pharmacol Ther 28: 235–246
Spector W (1956) Handbook of biological data. Saunders, Philadelphia, p 279
Schaaf L, Ferry D, Hung C, Perrier D, Edwards IR (1985) Analysis of 5′-deoxy-5-fluorouridine and 5-fluorouracil in human plasma and urine by high-performance liquid chromatography. J Chromatogr 342: 303–313
Benet L, Galeazzi R (1979) Noncompartmental determination of the steady-state volume of distribution. J Pharm Sci 68: 1071–1074
Perrier D, Mayersohn M (1982) Noncompartmental determination of the steady-state volume of distribution for any mode of administration. J Pharm Sci 71: 372–373
Yamaoka K, Nakagawa T, Uno T (1978) Statistical moments in pharmacokinetics. J Pharmacokinet Biopharm 6: 547–558
Lam F, Longnecker M (1983) A modified Wilcoxon rank sum test for paired data. Biometrika 70: 510–513
Bradley S, Ingelfinger F, Bradley G (1952) Hepatic circulation in cirrhosis of the liver. Circulation 5: 419–429
Finch R, Bending M, Lant A (1979) Plasma levels of 5-fluorouracil after oral and intravenous administration in cancer patients. Br J Clin Pharmacol 7: 613–617
Cadman E, Heimer R, Davis L (1979) Enhanced 5-fluorouracil nucleotide formation after methotrexate administration: Explanation for drug synergism. Science 205: 1135–1137
Garrett E, Hurst G, Green J Jr (1977) Kinetics and mechanisms of drug action on microorganisms XXIII: Microbial kinetic assay for fluorouracil in biological fluids and it's application to human pharmacokinetics. J Pharm Sci 66: 1422–1429
Wagner J, Gyves J, Stetson P, Walker-Andrews S, Wollner I, Cochran M, Ensminger W (1986) Steady-state nonlinear pharmacokinetics of 5-fluorouracil during hepatic arterial and intravenous infusions in cancer patients. Cancer Res 46: 1499–1506
Gibaldi M, Perrier D (1982) Nonlinear pharmacokinetics. In: Swarbrick J (ed) Pharmacokinetics, 2nd edn. Marcel Dekker, New York
Perrier D, Ashley J, Levy G (1973) Effect of product inhibition on kinetics of drug elimination. J Pharmacokinet Biopharm 1: 231–242
Mukherjee K, Heidelberger C (1960) Studies on fluorinated pyrimidines. IX. The degradation of 5-fluorouracil-6-C14. J Biol Chem 235: 433–437
Aubert C, Cano J, Rigault J, Seitz J, Carcassonne Y (1981) Pharmacokinetics of 5-fluorouracil: Impact of the measurement of the 5,6-dihydrofluorouracil. Bull Cancer 68: 343–345
Aubert C, Sommadossi J, Coasslo P, Cano J, Rigault J (1982) Quantitative analysis of 5-fluorouracil and 5,6-dihydrofluorouracil in plasma by gas chromatography mass spectrometry. Biomed Mass Spectrom 9: 336–339
Benet L, Massoud N (1984) Pharmacokinetics. In: Benet L, Massoud N, Gambertoglio J (eds) Pharmacokinetic basis for drug treatment. Raven Press, New York
Jusko W, Gibaldi M (1972) Effects of change in elimination on various parameters of the two-compartment open model. J Pharm Sci 61: 1270–1273
Celio L, DiGregorio G, Ruch E, Pace J, Piraino A (1983) Doxorubicin and 5-fluorouracil plasma concentrations and detectability in parotid saliva. Eur J Clin Pharmacol 24: 261–266
Diem K, Lentner C (eds) (1970) In: Documenta Geigy, 7th edn. Ciba-Geigy, Basle, Switzerland
Lam F, Hung C, Perrier D (1985) Estimation of variance for harmonic mean half-lives. J Pharm Sci 74: 229–231
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schaaf, L.J., Dobbs, B.R., Edwards, I.R. et al. Nonlinear pharmacokinetic characteristics of 5-fluorouracil (5-FU) in colorectal cancer patients. Eur J Clin Pharmacol 32, 411–418 (1987). https://doi.org/10.1007/BF00543978
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00543978