Summary
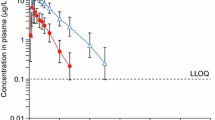
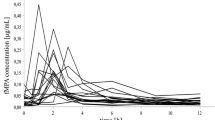
The disposition and plasma binding of methylprednisolone were examined in seven normal volunteers following the administration of 5, 20 and 40 mg of intravenous methylprednisolone sodium succinate. Methylprednisolone exhibits linear plasma protein binding averaging 77%. The mean plasma methylprednisolone clearance of 337 ml·h−1. kg−1 was independent of dose. The steroid appears to moderately distribute into tissue spaces with a mean volume of distribution of 1.41·kg−1. Methylprednisolone disposition parameters were compared with the non-transcortin bound parameters for prednisolone. The prednisolone plasma clearance based on the transcortin free-drug is similar to methylprednisolone total plasma clearance. However, the corrected volume of distribution of prednisolone is only one-half that of methylprednisolone. The disposition rate of these two steroids is thus similar, in spite of their metabolic control by different enzymatic pathways and major influence of saturable transcortin binding on prednisolone elimination.
Similar content being viewed by others
References
Melby JC (1977) Clinical pharmacology of systemic corticosteroids. Ann Rev Pharmacol Toxicol 17: 511–527
Baxter JD, Forsham PH (1972) Tissue effects of glucocorticoids. Am J Med 53: 573–589
Lewis GP, Jusko WJ, Burke CW, Groves L (1971) The Boston Collaborative Drug Surveillance Program: Prednisolone side-effects and serum protein levels. Lancet 2: 779–781
Kozower M, Veatch L, Kaplan MM (1974) Decreased clearance of prednisolone, a factor in the development of corticosteroid side-effects. J Clin Endocrinol Metab 38: 407–412
Meikle AW, Weed JA, Tyler FH (1975) Kinetics and interconversion of prednisolone and prednisone studied with new radioimmunoassays. J Clin Endocrinol Metab 41: 717–721
Pickup ME, Lose JR, Leatham PA, Rhind VM, Wright V, Downie WW (1977) Dose dependent pharmacokinetics of prednisolone. Eur J Clin Pharmacol 12: 213–219
Rose JQ, Yurchak AM, Jusko WJ (1981) Dose dependent pharmacokinetics of prednisone and prednisolone in man. J Pharmacokinet Biopharm 9: 389–417
Legler UF, Frey FJ, Benet LZ (1982) Prednisone clearance at steady-state in man. J Clin Endocrinol Metab 55: 762–767
Haynes RC, Larner J (1975) Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of adrenocortical steroid biosynthesis. In: Goodman LS, Gilman A (eds) The pharmacologic basis of therapeutics, fifth edition. MacMillan, New York, p 1491
Ballard PL (1979) In: Monographs in endocrinology 12. Baxter JD, Rousseau GG (eds) Glucocorticoid hormone action. Springer, Heidelberg, pp 25–45
Ebling WF, Szefler SJ, Jusko WJ (1983) Analysis of cortisol, methylprednisolone, and its succinate ester: Absence of effects of troleandomycin on ester hydrolysis. J Chromatogr Biomed Applic 305: 271–280
Ebling WF, Milsap RL, Szefler SJ, Jusko WJ (1985) Determination of methylprednisolone and methylprednisolone binding in human and rabbit plasma. J Pharm Sci (in press)
Boudinot FD, Jusko WJ (1984) Fluid shifts and other factors affecting plasma protein binding of prednisolone by equilibrium dialysis. J Pharm Sci 73: 774–780
Rocci ML, Jusko WJ (1983) LAGRAN program for area and moments in pharmacokinetic analysis. Comput Progr Biomed 16: 203–216
Metzler CM, Elfring GL, McEwen (1974) NONLIN, A computer program for nonlinear least-squares regression. Biometrics 30: 562
Jusko WJ (1980) Guidelines for collection and pharmacokinetic analysis of drug disposition data. In: Evans WE, Schentag JJ, Jusko WJ (eds) Applied pharmacokinetics: Principles of therapeutic drug monitoring. Applied Therapeutics, San Francisco, p 639
Bruning JL, Kintz BL (1977) Treatment-by-subjects, or repeated measures, design. Computational handbook of statistics, second edition. Scott, Foresman, Glenview, IL, pp 44–48
Wagner JG (1975) Fundamentals of clinical pharmacokinetics, first edition. Hamilton, IL, Drug Intelligence, p 286
Pardridge WM, Mietus LJ (1979) Transport of protein-bound hormones into liver in vivo. Am J Physiol 237: E367-E372
Ebling WF, Szefler SJ, Jusko WJ (1985) Methylprednisolone disposition in rabbits: Analysis, prodrug conversion, reversible metabolism, and comparison with man. Drug Metab Dispos 13: 296–304
Davis M, Williams R, Chakraboty J, Marks V, Ideo G, Tempini S (1978) Prednisone or prednisolone for the treatment of chronic active hepatitis? A comparison of plasma availability. Br J Clin Pharmacol 5: 501–505
MacDonald IA, Bokkenheuser VD, Winter J, McLernon AM, Mosbach EH (1983) Degradation of steroids in the human gut. J Lipid Res 24: 675–700
Assael BM, Banfi AC, Edefonti A, Jusko WJ (1982) Disposition of pulse dose methylprednisolone in adult pediatric patients with the nephrotic syndrome. Eur J Clin Pharmacol 23: 429–433
Leo A, Hansch C, Elkins D (1971) Partition coefficients and their uses. Chem Rev 71: 525–616
Tomkins GM (1959) Enzymatic metabolism of corticosteroids. Ann NY Acad Sci 82: 836–853
Bush IE, Hunter SA, Meigs RA (1968) Metabolism of 11-oxygenated steroids: Metabolism in vitro by preparations in liver. Biochem J 107: 239–258
Vermeulen A, Caspi E (1959) Metabolism of 1-dehydrosteroids in man. II. Isolation of 20 alpha- and 20 beta-metabolites. J Biol Chem 234: 2295–2297
Elias AN, Gwinup G (1980) Effects of some clinically encountered drugs on steroid synthesis and degradation. Metabolism 29: 582–595
Saenger P (1983) 6-Beta-hydrocortisol in random urine samples as an indicator of enzyme induction. Clin Pharmacol Ther 34: 818–821
Szefler SJ, Ellis EF, Brenner M, Rose JQ, Spector SL, Yurchak AM, Andrews F, Jusko WJ (1982) Steroid-specific and anticonvulsant interaction aspects of troleandomycin-steroid therapy. J Allergy Clin Immunol 69: 455–460
Pessayre D, Konstantinova-Mitcheva, Descatoire V, Colbert B, Wandscheer J, Level R, Feldmann G, Mansuy D, Benhamou P (1981) Hypoactivity of cytochrome P-450 after triacetyloleandomycin administration. Biochem Pharmacol 30: 559–564
Braude AC, Rebuck AS (1983) Prednisone and methylprednisolone disposition in the lung. The Lancet 2: 995–997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Szefler, S.J., Ebling, W.F., Georgitis, J.W. et al. Methylprednisolone versus prednisolone pharmacokinetics in relation to dose in adults. Eur J Clin Pharmacol 30, 323–329 (1986). https://doi.org/10.1007/BF00541537
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00541537