Summary
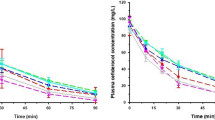
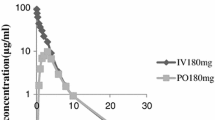
The purpose of this study was to investigate the pharmacokinetics of intraperitoneally (IP) administered ceftriaxone (CRO) in patients maintained on chronic peritoneal dialysis. A single 2 g dose of CRO was administered IP to six adult patients who did not have peritonitis at the time of study. After a 5 hour dwell, the peritoneal fluid was exchanged with CRO-free fluid. Exchanges were carried out every 4 to 8 h, over a 24- to 28-h period. The peak total plasma CRO concentration was 104 µg/ml. An average of 74.1% of the IP dose of CRO was absorbed. Plasma protein binding was nonlinear; mean free fraction ranged from 12.8 to 17.9% at low and high concentrations. Dialysate concentrations at the end of subsequent exchanges ranged from means of 19.9 to 2.9 µg/ml. Total CRO clearance from plasma was 10.1 ml·kg−1·h−1 and the mean terminal t1/2 was 12.7 h. Dialytic clearance averaged 0.69 ml·kg−1·h−1, only 6.9% of total clearance. A model which incorporates known characteristics of CRO binding and distribution in anuric patients was used to simulate plasma and peritoneal concentrations of CRO during multiple dose IP drug administration.
Similar content being viewed by others
References
Popovich RP, Moncrief JW, Decherd JB, Bamar JB, Pyle WK (1976) The definition of a novel portable/wearable equilibrium peritoneal dialysis technique. Abstracts Am Soc Art Int Org 5: 64
Council report (1981) Continuous ambulatory peritoneal dialysis. J Am Med Assoc 248: 2340–2341
Rubin J, Robers WA, Taylor HM, Everett ED, Prowant BF, Fruto DV, Nolph KD (1980) Peritonitis during continuous ambulatory peritoneal dialysis. Ann Intern Med 92: 7–13
Oreopoulus OG, Robson M, Faller B, Ogilvie R, Rapaport A, DeVeber GA (1979) Continuous ambulatory peritoneal dialysis: A new era in the treatment of chronic renal failure. Clin Nephrol 11: 125–128
Angehrn P, Probst PJ, Reiner R, Then RL (1980) Ro 13-9904, a long-acting broad spectrum cephalosporin: In vitro and in vivo studies. Antimicrob Agents Chemother 18: 913–921
Steinhauer HB, Gunter B, Schollmeyer P (1983) Peritonitis bei kontinuierlicher ambulanter Peritonealdialyse (CAPD): Untersuchungen über die Freisetzung von Arachidonsäuremetaboliten. Verh Dtsch Ges Inn Med 89: 965–968
Stoeckel K, McNamara PJ, Hoppe Seyler G, Blumberg A, Keller E (1983) Single-dose certriaxone kinetics in functionally anephric patients. Clin Pharmacol Ther 33: 633–641
Trautmann KH, Haefelfinger P (1981) Determination of Ro 13-9904 in plasma, urine and bile by means of ion-pair reversed phase chromatography. J High Resol Chromatogr Commun 4: 54–59
Coffey JJ, Bullock FJ, Schoenemann PT (1971) Numerical solution of nonlinear pharmacokinetic equations: Effect of plasma protein binding on drug distribution and elimination. J Pharm Sci 60: 1623–1628
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn, Marcel Dekker, Basel, New York
Stoeckel K, Koup JR (1984) Pharmacokinetics of ceftriaxone in patients with renal and liver insufficiency and correlations with a physiologic nonlinear protein binding model. Am J Med 77 (4c): 26–32
Stoeckel K, McNamara PJ, Brandt R, Plozza-Nottebrock H, Zeigler WH (1981) The effects of concentration dependent plasma protein binding on the pharmacokinetics of ceftriaxone, a new parenteral cephalosporin. Clin Pharmacol Ther 29: 650–657
Ti T-Y, Fortin L, Kreeft JH, East DS, Ogilvie RI, Somerville PJ (1984) Kinetic disposition of intravenous ceftriaxone in normal subjects and patients with renal failure on hemodialysis or peritoneal dialysis. Antimicrob Agents Chemother 25: 83–87
Flessner MF, Dedrick RL, Schultz JR (1984) A distributed model of peritoneal-plasma transport: theoretical considerations. Am J Physiol 246: R597–607
Cleeland R, Squires E (1984) Antimicrobial activity of ceftriaxone: A review. Am J Med 77 (4c): 3–11
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koup, J.R., Keller, E., Neumann, H. et al. Ceftriaxone pharmacokinetics during peritoneal dialysis. Eur J Clin Pharmacol 30, 303–307 (1986). https://doi.org/10.1007/BF00541533
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00541533