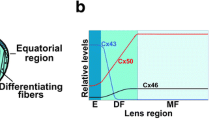
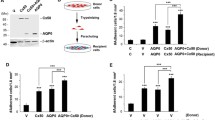
Abstract
Lens and liver contain many gap junctions, which for a long time have been considered to be very similar. Recent results, however, point to differences on morphological and biochemical levels, especially when the liver gap junction polypeptide (26,000 Daltons) is compared with the main intrinsic polypeptide (MIP) from lens junctions. The lens fiber specific MIP, which represents a marker molecule for lens cell differentiation could be detected by indirect immunofluorescence as well as by immunodiffusion in lens epithelial cells, which differentiated in vitro under distinct culture conditions. The fine structure of these differentiated cells is presented.
Similar content being viewed by others
References
Bloemendal H, Vermorken AJM, Kibbelaar M, Dunia I, Benedetti EL (1977) Nomenclature for the polypeptide chains of lens plasma membranes. Exp Eye Res 24: 413–415
Bok D, Dockstader J, Horwitz J (1982) Immunocytochemical localization of the lens main intrinsic polypeptide (MIP 26) in communicating junctions. J Cell Biol 92: 213–220
Broekhuyse RM, Kuhlmann ED, Stols AD (1976) Lens membranes II. Isolation and characterization of the main intrinsic polypeptide (MIP) of bovine lens fiber membranes. Exp Eye Res 23: 365–371
Broekhuyse RM, Kuhlmann ED, Winkens HJ (1979) Lens membranes VII, MIP is an immunological specific component of lens fiber membranes and is identical with 26 K band protein. Exp Eye Res 29: 303–313
Dell'Orco RT (1975) The use of arrested populations of human diploid fibroblasts for the study of senescence in vitro. Adv Exp Med Biol 53: 41–51
Goodenough DA, Dick JSB, Lyons JE (1980) Lens metabolic cooperation: a study of mouse lens transport and permeability visualized with freeze substitution autoradiography and electron microscopy. J Cell Biol 86: 576–589
Henderson D, Eibl H, Weber K (1979) Structure and biochemistry of hepatic gap junctions. J Mol Biol 132: 193–218
Hertzberg EL (1980) Biochemical and immunocytochemical approaches to the study of gap junctional communication. In Vitro 16: 1057–1067
Hertzberg EL, Anderson DJ, Friedlander M, Gilula NB (1982) Comparative analysis of the major polypeptides from liver gap junctions and lens fiber junctions. J Cell Biol 92: 53–59
Hockwin O (1971) Age changes of lens metabolism. In: Brecht H, Rohen JW (eds) Altern und Entwicklung. Schattauer Verlag, Stuttgart, pp 95–129
Horwitz J, Wong MM (1980) Peptide mapping by limited proteolysis in sodiumdodecylsulphate of the main intrinsic polypeptides isolated from human and bovine lens plasma membranes. Biochim Biophys Acta 622: 134–143
Kubawara T (1975) The maturation of the lens cell. A morphologic study. Exp Eye Res 20: 427–443
Nicholson BJ, Hunkapiller MW, Hood LE, Revel JP, Takemoto L (1980) Partial sequencing of the gap junction protein from rat lens and liver. J Cell Biol 87: 1539a (Abstract)
Peracchia C, Peracchia LL (1980) Gap junction dynamics: reversible effects of hydrogen ions. J Cell Biol 87: 719–727
Rae JL (1979) The electrophysiology of the crystalline lens. In: Zadunaisky JA, Davson H (eds) Current topics in eye research. Academic Press, New York, pp 37–90
Rafferty NS, Esson EA (1974) An electron microscope study of adult mouse lens: some ultrastructural specializations. J Ultrastruct Res 46: 239–253
Rink H (1978) The water content of bovine lenses during aging. Interdiscip Top Gerontol 12: 272–277
Rink H, Vornhagen R (1980) Crystallins of rat and bovine lens epithelial cells during aging. In: Regnault F, Hockwin O, Courtois Y (eds) Aging of the lens. Elsevier, North-Holland, Amsterdam, pp 37–51
Takemoto LJ, Hansen JS, Horwitz J (1981) Interspecies conservation of the main intrinsic polypeptide (MIP) of the lens membrane. Comp Biochem Physiol 68B: 101–106
Traub O, Janßen-Timmen U, Drüge P, Dermietzel R, Willecke K (1982) Immunological properties of gap junction protein from mouse liver. J Cell Biochem 19: 27–43
Vornhagen R, Rink H (1981) The expression of crystallins in early and late passages of calf lens epithelial cells grown under different culture conditions. J Cell Biol 24: 25
Vornhagen R, Rink H, Broekhuyse RM (1982) Main intrinsic polypeptide (MIP) a membrane protein as marker molecule for lens cell differentiation. J Cell Biol 27: 33
Willecke K, Traub O, Janßen-Timmen U, Drüge P, Dermietzel R (1982) Expression of gap junction protein in liver and lens tissue. In: Jaenicke L (ed) Biochemistry of differentiation and morphogenesis. Springer, Berlin Heidelberg New York
Zampighi G, Simon SA, Robertson JD, McIntosh TJ, Costello MJ (1982) On the structural organization of isolated bovine lens fiber junctions. J Cell Biol 93: 175–189
Zigler JS, Horwitz J (1981) Immunochemical studies on the major intrinsic membrane polypeptide from human lens. Invest Ophthalmol 21: 46–51
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rink, H. The major polypeptide (MIP) of lens fiber junctions and its synthesis in cultured differentiating lens epithelial cells. Biophys. Struct. Mechanism 9, 95–101 (1982). https://doi.org/10.1007/BF00539107
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00539107