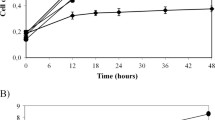
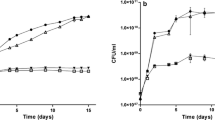
Summary
In Rhizobium meliloti the NifA protein plays a central role in the expression of genes involved in nitrogen fixation. The R. meliloti NifA protein has been found to be oxygen sensitive and therefore acts as a transcriptional activator only under microaerobic conditions. In order to generate oxygen-tolerant variants of the NifA protein a plasmid carrying the R. meliloti nifA gene was mutagenized in vitro with hydroxylamine. About 70 mutated nifA genes were isolated which mediated up to 12-fold increased NifA activity at high oxygen concentrations. A cloning procedure involving the combination of DNA fragments from mutated and wild-type nifA genes allowed mapping of the mutation sites within the central part of the nifA gene. For 17 mutated nifA genes the exact mutation sites were determined by DNA sequence analysis. It was found that all 17 mutated nifA genes carried identical guanosine — adenosine mutations resulting in a methionine — isoleucine exchange (M217I) near the putative nucleotide binding site within the central domain. Secondary structure predictions indicated that the conformation of the putative nucleotide binding site may be altered in the oxygen-tolerant NifA proteins. A model is proposed which assumes that at high oxygen concentrations the loss of activity of the R. meliloti NifA protein is due to a conformational change in the nucleotide binding site that may abolish binding or hydrolysis of the nucleotide. Such a conformational change may be blocked in the oxygen-tolerant NifA protein, thus allowing interaction with the nucleotide at high oxygen concentrations.
Similar content being viewed by others
References
Argos P, Hanei M, Garavito RM (1978) The Chou-Fasman secondary structure prediction method with an extended data base. FEBS Lett 93:19–24
Austin S, Kundrot C, Dixon R (1991) Influence of a mutation in the putative nucleotide binding site of the nitrogen regulatory protein NTRC on its positive control function. Nucleic Acids Res 19:2281–2287
Ausubel FM (1984) Regulation of nitrogen fixation genes. Cell 37:5–6
Beaucage SL, Caruthers MH (1981) Deoxynucleoside phosphoramidites — a new class of key intermediates for deoxypolynucleotide synthesis. Tetrahedron Lett 22:1859–1862
Bennett LT, Cannon F, Dean DR (1988) Nucleotide sequence and mutagenesis of the nifA gene from Azotobacter vinelandii. Mol Microbiol 2:315–321
Berg JM (1986) Potential metal-binding domains in nucleic acid binding proteins. Science 232:485–487
Beynon JL, Williams MK, Cannon FC (1988) Expression and functional analysis of the Rhizobium meliloti nifA gene. EMBO J 7:7–14
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Buck M, Miller S, Drummond M, Dixon R (1986) Upstream activator sequences are present in the promoters of nitrogen fixation genes. Nature 320:374–378
Buikema WJ, Szeto WW, Lemley PV, Orme-Johnson WH, Ausubel FM (1985) Nitrogen fixation specific regulatory genes of Klebsiella pneumoniae and Rhizobium meliloti share homology with the general nitrogen regulatory gene ntrC of K. pneumoniae. Nucleic Acids Res 13:4539–4555
Chou PY, Fasman GD (1974a) Conformational parameters for amino acids in helical, β-sheet, and random coil regions calculated from proteins. Biochemistry 13:211–222
Chou PY, Fasman GD (1974b) Prediction of protein conformation. Biochemistry 13:222–245
DeFeo-Jones D, Scolnick EM, Koller R, Dhar R (1983) ras-related gene sequences identified and isolated from Saccharomyces cerevisiae. Nature 306:707–709
Dixon R, Eydmann T, Henderson N, Austin S (1991) Substitutions at a single amino acid residue in the nitrogen-regulated activator protein NTRC differentially influence its activity in response to phosphorylation. Mol Microbiol 5:1657–1667
Drummond M, Whitty P, Wootton J (1986) Sequence and domain relationships of ntrC and nifA from Klebsiella pneumoniae: homologies to other regulatory proteins. EMBO J 5:441–447
Drummond MH, Contreras A, Mitchenall LA (1990) The function of isolated domains and chimaeric proteins constructed from the transcriptional activators NifA and NtrC of Klebsiella pneumoniae. Mol Microbiol 4:29–37
Fischer H-M, Bruderer T, Hennecke H (1988) Essential and nonessential domains in the Bradyrhizobium japonicum NifA protein: identification of indispensable cysteine residues potentially involved in redox reactivity and/or metal binding. Nucleic Acids Res 16:2207–2224
Fischer H-M, Fritsche S, Herzog B, Hennecke H (1989) Critical spacing between two essential cysteine residues in the interdomain linker of the Bradyrhizobium japonicum NifA protein. FEBS Lett 255:167–171
Fry DC, Kuby SA, Mildvan AS (1986) ATP-binding site of adenylate kinase: Mechanistic implications of its homology with ras-encoded p21, F1-ATPase, and other nucleotide-binding proteins. Proc Natl Acad Sci USA 83:907–911
Garnier J, Osguthorpe DJ, Robson B (1978) Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol 120:97–120
Grönger P, Manian SS, Reiländer H, O'Connell M, Priefer UB, Pühler A (1987) Organization and partial sequence of a DNA region of the Rhizobium leguminosarum symbiotic plasmid pRL6JI containing the genes fixABC, nifA, nifB and a novel open reading frame. Nucleic Acids Res 15:31–49
Gussin GN, Robson CW, Ausubel FM (1986) Regulation of nitrogen fixation genes. Annu Rev Genet 20:567–591
Henderson N, Austin S, Dixon RA (1989) Role of metal ions in negative regulation of nitrogen fixation by the nifL gene product from Klebsiella pneumoniae. Mol Gen Genet 216:484–491
Hill S, Kennedy C, Kavanagh E, Goldberg RB, Hanau R (1981) Nitrogen fixation gene (nifL) involved in oxygen regulation of nitrogenase synthesis in K. pneumoniae. Nature 290:424–426
Hirschmann J, Wong P-K, Sci K, Keener J, Kustu S (1985) Products of nitrogen regulatory genes ntrA and ntrC of enteric bacteria activate glnA transcription in vitro: Evidence that the ntrA product is a σ factor. Proc Natl Acad Sci USA 82:7525–7529
Huala E, Ausubel FM (1989) The central domain of Rhizobium meliloti NifA is sufficient to activate transcription from the R. meliloti nifH promoter. J Bacteriol 171:3354–3365
Huala E, Moon AL, Ausubel FM (1991) Aerobic inactivation of Rhizobium meliloti NifA in Escherichia coli is mediated by lon and two newly identified genes, snoB and snoC. J Bacteriol 173:382–390
Hunt TP, Magasanik B (1985) Transcription of glnA by purified Escherichia coli components: Core RNA polymerase and the products of glnF, glInG and glnL. Proc Natl Acad Sci USA 82:8453–8457
Iismaa SE, Watson JM (1989) The nifA gene product from Rhizobium leguminosarum biovar trifolii lacks the N-terminal domain found in other NifA proteins. Mol Microbiol 3:943–955
Jurnak F (1985) Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science 230:32–36
Klipp W, Reiländer H, Schlüter A, Krey R, Pühler A (1989) The Rhizobium meliloti fdxN gene encoding a ferredoxin-like protein is necessary for nitrogen fixation and is cotranscribed with nifA and nifB. Mol Gen Genet 216:293–302
Kuby SA, Palmieri RH, Frischat A, Fischer AH, Wu LH, Maland L, Manship M (1984) Studies on adenosine triphosphate transphosphorylases. Amino acid sequence of rabbit muscle ATP-AMP transphosphorylase. Biochemistry 23:2393–2399
Laursen RA, L'Italien JJ, Nagarkatti S, Miller DL (1981) The amino acid sequence of elongation factor Tu of Escherichia coli. J Biol Chem 256:8102–8109
MacNeil D (1981) General method, using Mu-Mud1 dilysogens, to determine the direction of transcription of and generate deletions in the glnA region of Escherichia coli. J Bacteriol 146:260–268
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual.Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Masepohl B, Klipp W, Pühler A (1988) Genetic characterization and sequence analysis of the duplicated nifA/nifB gene region of Rhodobacter capsulatus. Mol Gen Genet 212:27–37
Matteucci MD, Caruthers MH (1981) Synthesis of deoxyoligonucleotides on a polymer support. J Am Chem Soc 103:3185–3191
Merrick M, Hill S, Hennecke H, Hahn M, Dixon R, Kennedy C (1982) Repressor properties of the nifL gene product in Klebsiella pneumoniae. Mol Gen Genet 185:75–81
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Möller W, Amons R (1985) Phosphate-binding sequences in nucleotide-binding proteins. FEBS Lett 186:1–7
Moreno-Vivian C, Schmehl M, Masepohl B, Arnold W, Klipp W (1989) DNA sequence and genetic analysis of the Rhodobacter capsulatus nifENX gene region: Homology between NifX and NifB suggests involvement of NifX in processing of the ironmolybdenum cofactor. Mol Gen Genet 216:353–363
Morett E, Buck M (1989) In vivo studies on the interaction of RNA polymerase-σ54 with the Klebsiella pneumoniae and Rhizobium meliloti nifH promoters. The role of NifA in the formation of an open promoter complex. J Mol Biol 210:65–77
Morett E, Cannon W, Buck M (1988) The DNA-binding domain of the transcriptional activator protein NifA resides in its carboxy terminus, recognises the upstream activator sequences of nif promoters and can be separated from the positive control function of NifA. Nucleic Acids Res 16:11469–11488
Morett E, Fischer H-M, Hennecke H (1991) Influence of oxygen on DNA binding, positive control, and stability of the Bradyrhizobium japonicum NifA regulatory protein. J Bacteriol 173:3478–3487
Nees DW, Stein PA, Ludwig RA (1988) The Azorhizobium caulinodans nifA gene: identification of upstream-activating sequences including a new element, the “anaerobox”. Nucleic Acids Res 16:9839–9853
Pai EF, Kabsch W, Krengel U, Holmes KC, John J, Wittinghofer A (1989) Structure of the guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in the triphosphate conformation. Nature 341:209–214
Popham DL, Szeto D, Keener J, Kustu S (1989) Function of a bacterial activator protein that binds to transcriptional enhancers. Science 243:629–635
Pühler A, Klipp W, Weber G (1983) Mapping and regulation of the structural genes nifK, nifD, and nifH of Rhizobium meliloti. In: Pühler A (ed) Molecular genetics of the bacteria-plant interaction. Springer-Verlag, Berlin-Heidelberg, pp 69–78
Roelvink PW, Hontelez JGJ, van Kammen A, van den Bos RC (1989) Nucleotide sequence of the regulatory nifA gene of Rhizobium leguminosarum PRE: transcriptional control sites and expression in Escherichia coli. Mol Microbiol 3:1441–1447
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Saraste M, Sibbald PR, Wittinghofer A (1990) The P-loop —a common motif in ATP- and GTP-binding proteins. TIBS 15:430–434
Souza EM, Funayama S, Rigo LU, Yates MG, Pedrosa FO (1991) Sequence and structural organization of a nifA-like gene and part of a nifB-like gene of Herbaspirillum seropedicae strain Z78. J Gen Microbiol 137:1511–1522
Thöny B, Fischer H-M, Anthamatten D, Bruderer T, Hennecke H (1987) The symbiotic nitrogen fixation regulatory operon (fzxRnifA) of Bradyrhizobium japonicum is expressed aerobically and is subject to a novel, nifA-independent type of activation. Nucleic Acids Res 15:8479–8499
Vieira J, Messing J (1982) The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259–268
Weber G, Pühler A (1982) Expression of Rhizobium meliloti genes for nitrogen fixation in Escherichia coli minicells: Mapping of the subunit of the nitrogenase reductase. Plant Molecular Biology 1:305–320
Weber G, Reilander H, Pühler A (1985) Mapping and expression of a regulatory nitrogen fixation gene (fixD) of Rhizobium meliloti. EMBO J 4:2751–2756
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krey, R., Pülder, A. & Klipp, W. A defined amino acid exchange close to he putative nucleotide binding site is responsible for an oxygen-tolerant variant of the Rhizobium meliloti NifA protein. Molec. Gen. Genet. 234, 433–441 (1992). https://doi.org/10.1007/BF00538703
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00538703