Abstract
-
1)
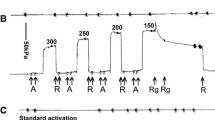
A study is presented on the effect of temperature on the mechanochemical states involved in the cross bridge cycle of single glycerinated dorsal longitudinal fibres from Lethocerus. Contraction was induced by immersing the fibre in a MgATP-salt solution at Ca2+∼10 ΜM (pH 6.7).
-
2)
Rising the temperature increases the rates of isometric tension generation following an increase in the [Ca2+] from 0.01 to 10 ΜM as well as the steady state levels of isometric tension and the rates of ATP splitting.
-
3)
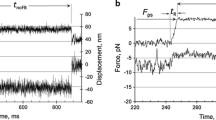
Tension transients following stretches of rise times 250 Μs and amplitudes up to 0.4% L i comprise at least four phases: an elastic phase the amplitude of which decreases by raising the temperature; a biphasic quick phase of tension decay with a mean Q 10=2; a delayed tension rise (Q 10∼5).
-
4)
Tension transients following releases of fall time 250 Μs and amplitudes up to 0.3% L i also comprise four phases: an elastic phase comparable to that observed following stretches; a deactivation phase composed of a single exponential and a slow recovery phase.
-
5)
The number of cross bridges attached to the actin at any moment is not changed during the elastic and quick recovery phase following a release as well as during the elastic and fast quick phase following a stretch. However, the number of attached cross bridges decreases during the deactivation phase.
-
6)
The early phases of tension adjustment (T curves) which were recorded during the releases showed a marked dependence on temperature. The T curves fitted with high accuracy the Huxley and Simmons (1971) predictions of cross bridge rotation.
-
7)
Analysis of the T curves in terms of the Huxley and Simmons (1971) model shows that a) the stiffness of a single cross bridge (D=1.2 104− N/m) obeys Hook's law; b) the number of myosin heads attached to actin (24% of the total number) is not altered during releases; c) rotation of myosin heads from a perpendicular to an acute angled position extends the elastic element of a cross bridge by 11 nm; d) at 25‡ C the rate constants for rotation from the perpendicular position to the acute angled position and vice versa are 8300 s−1 (Q 10=3.5) and 3600 s−1 (Q 10=1.5).
-
8)
Thermodynamics applied to cross bridge rotation predicts that during sudden releases the temperature within the fibre should fall and the driving force for tension generation is an increase in entropy of rotated bridges.
-
9)
Rate constant of detachment of cross bridges from the actin is determined to k 2=500 s−1 (25‡ C; Q 10=2.3).
-
10)
The values of steady state rate of ATP splitting in conjunction with estimates of the number of attached cross bridges indicate that rate limiting steps of ATP cleavage occur while myosin heads are detached and while they are attached to the actin: Rate limiting for the attachment is the decay of the refractory myosin-product (Eisenberg and Kielley, 1973; k 4=1.7 s−1: 25‡ C; Q 10=2.5). Rate limiting for detachment is the concentration ratio of actomyosin-ATP to actomyosin-product K 1=0.018 (25‡ C; Q 10=2.7).
Similar content being viewed by others
References
Abbott, R. H.: An interpretation of the effects of fibre length and calcium on the mechanical properties of insect flight muscle. Cold Spring Harbor Symp. Quant. Biol. 37, 647–654 (1973)
Abbott, R. H., Steiger, G. J.: Temperature and amplitude dependence of tension transients in glycerinated skeletal and insect fibrillar muscle. J. Physiol. 266, 13–42 (1977)
Armitage, P., Miller, A., Rodger, C. D., Tregear, R. T.: The structure and function of insect muscle. Cold Spring Harbor Symp. Quant. Biol. 37, 379–387 (1973)
Ashley, C. C., Moisescu, D. G.: The part played by Ca2+ in the contraction of isolated bundles of myofibrils. In: Calcium transport in contraction and secretion. Carafoli, E. (ed.), pp. 517–525. Amsterdam: North Holland 1975
Bagshaw, C. R., Trentham, D. R.: The reversibility of adenosine triphosphate cleavage by myosin. Biochem. J. 133, 323–328 (1973)
Barrington Leigh, J., Goody, R. S., Hofmann, W., Holmes, K., Mannherz, H. G., Rosenbaum, G., Tregear, R. T.: The interpretation of X-ray diffraction from glycerinated flight muscle fibre bundles: new theoretical and experimental approaches. In: Symposium on insect flight muscle, Oxford 1977. Tregear, R. T. (ed.), pp. 137–146. Amsterdam: Elsevier/North Holland 1977
Beinbrech, G., Kuhn, H. J., Herzig, J. W., Rüegg, J. C.: Evidence for two attached myosin cross bridges states of different potential energy. Cytobiology 12, 385–396 (1976)
Benoit, H.: Calcul de l'écart quadratique moyen entre les extrémités de diverses chaÎnes moléculaires de type usuel. J. Polymer Sci. 3, 376–388 (1948)
Breull, W., Steiger, G., Rüegg, J. C.: ATP splitting in relation to isometric tension-oscillation and cross bridge cycling of insect fibrillar muscle. J. Mechanochem. Cell Motil. 2, 91–100 (1973)
Chock, S. P., Chock, P. B., Eisenberg, E.: Pre-steady-state kinetic evidence for a cyclic interaction of myosin subfragment one with actin during the hydrolysis of adenosine 5′-Triphosphate. Biochemistry 15, 3244–3253 (1976)
Davies, R. E.: A molecular theorie of muscle contraction: calcium dependent contractions with hydrogen bond formation plus ATP-dependent extensions of part of the myosin cross bridges. Nature (Lond.) 199, 1068–1074 (1962)
Doty, P., Wada, A., Yang, J. T., Blout, E. R.: Polypeptides. VIII. Molecular configurations of poly-L- glutamic acid in water-dioxane solution. J. Polymer Sci. 23, 851–861 (1957)
Eisenberg, E., Hill, T. L.: A cross bridge model of muscle contraction. Prog. Biophys. Mol. Biol. 33, 55–82 (1978)
Eisenberg, E., Kielley, W. W.: Evidence for a refractory state of heavy meromyosin and subfragment-1 unable to bind to actin in the presence of ATP. Cold Spring Harbor Symp. Quant. Biol. 37, 443–447 (1973)
Feldhaus, P., Fröhlich, T., Goody, R. S., Isakov, M., Schirmer, R. H.: Synthetic inhibitors of adenylate kinases in the assays for ATPases and phosphokinases. Eur. J. Biochem. 57, 197–204 (1975)
Flitney, F. W., Hirst, D. G.: Cross bridge detachment and sarcomere “give” during stretch of active frog's muscle. J. Physiol. 276, 449–465 (1978)
Flory, P. J.: Role of crystallization in polymers and proteins. Science 124, 53–60 (1956)
Ford, L. E., Huxley, A. F., Simmons, R. M.: Mechanism of early tension recovery after a quick release in tetanized muscle fibres. J. Physiol. 240, 42P-43P (1974)
Ford, L. E., Huxley, A. F., Simmons, R. M.: The instantaneous elasticity of frog skeletal muscle fibres. J. Physiol. 260, 28–29P (1976)
Ford, L. E., Huxley, A. F., Simmons, R. M.: Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J. Physiol. 269, 441–515 (1977)
Goody, R. S., Holmes, K. C., Mannherz, H. G., Barrington Leigh, J., Rosenbaum, G.: X-ray diffraction studies of insect fight muscle with ATP analogues. Biophys. J. 15, 687–704 (1975)
Gordon, A., Huxley, A. F., Julian, F. J.: The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 184, 170–192 (1966)
Güth, K., Kuhn, H. J.: Cross bridge elasticity in the presence and absence of ATP. Pflügers Arch. 362, R25 (1976)
Güth, K., Kuhn, H. J.: Stiffness and tension during and after sudden length changes of glycerinated rabbit psoas muscle fibres. Biophys. Struct. Mech. 4, 223–236 (1978)
Güth, K., Kuhn, H. J., Drexler, B., Berberich, W., Rüegg, J. C.: Stiffness and tension during and after sudden length changes of glycerinated single insect fibrillar muscle fibres. Biophys. Struct. Mech. 5, 255–276 (1979)
Guth, E., Mark, H.: Zur innermolekularen Statistik, insbesondere bei Kettenmolekülen I. Monatsh. Chem. 65, 93–121 (1935)
Harrington, W. F.: A mechanochemical mechanism for muscle contraction. Proc. Natl. Acad. Sci. USA 68, 685–689 (1971)
Herzig, J. W.: A model of stretch activation based on stiffness measurements in glycerol extracted insect fibrillar flight muscle. In: Symposium on insect flight muscle, Oxford 1977. Tregear, R. T. (ed.), pp. 209–219. Amsterdam: Elsevier/North Holland 1977
Hill, A. V.: The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Ser. B 126, 136–211 (1938)
Hill, A. V.: The “instantaneous” elasticity of active muscle. Proc. R. Soc. Ser. B 141, 161 (1953)
Hill, T. L.: Theoretical formalism for the sliding filament model of contraction of striated muscle, Part I. Prog. Biophys. 28, 267–340 (1974)
Holmes, K. C.: The myosin cross bridge as revealed by structure studies. Int. Symp. Rottach-Egern Tegernsee 1976. Riecker, G. et al. (eds.). Berlin, Heidelberg, New York: Springer 1977
Huxley, A. F.: Muscle structure and theories of contraction. Prog. Biophys. 7, 255–318 (1957)
Huxley, A. F.: Muscular contraction. J. Physiol. 243, 1–43 (1974)
Huxley, A. F., Simmons, R. M.: A quick phase in the series-elastic component of striated muscle, demonstrated in isolated fibres from the frog. J. Physiol. 208, 52 (1970)
Huxley, A. F., Simmons, R. M.: Proposed mechanism of force generation in striated muscle. Nature 233, 535 (1971)
Huxley, A. F., Simmons, R. M.: Mechanical transients and the origin of muscular force. Cold Spring Harbor Symp. Quant. Biol. 37, 669–680 (1973)
Huxley, H. E.: The mechanism of muscular contraction. Science 164, 1356–1366 (1969)
Jewell, B. R., Rüegg, J. C.: Oscillatory contraction of insect fibrillar muscle after glycerol extraction. Proc. R. Soc. Ser. B 164, 428 (1966)
Julian, F. J.: Activation in a skeletal muscle contraction model with a modification for insect fibrillar muscle. Biophysic. J. 9, 547–570 (1969)
Julian, F. J., Sollins, K. R., Sollins, M. R.: A model for the transient and steady-state mechanical behaviour of contracting muscle. Biophys. J. 14, 546–562 (1974)
Kuhn, H. J.: Reversible transformation of mechanical work into chemical free energy by stretch-dependent binding of AMP-PNP in glycerinated fibrillar muscle fibres. In: Symposium on insect flight muscle, Oxford 1977. Tregean, R. T. (ed.), pp. 307–315. Amsterdam: Elsevier/North Holland 1977
Kuhn, H. J.: Tension transients in fibrillar muscle fibres as affected by stretch-dependent binding of AMP-PNP: A teinochemical effect? Biophys. Struct. Mech. 4, 209–222 (1978)
Kuhn, W.: über die Gestalt fadenförmiger Moleküle in Lösungen. Kolloid-Z. 68, 2–15 (1934)
Kuhn, W., Grün, F.: Beziehungen zwischen elastischen Konstanten und Dehnungsdoppelbrechung hochelastischer Stoffe. Kolloid-Z. 101, 248–271 (1942)
Kuhn, W., Ramel, A., Walters, D. H., Ebner, G., Kuhn, H. J.: The production of mechanical energy from different forms of chemical energy with homogeneous and cross-striated high polymer systems. Fortschr. Hochpolym. Forsch. 1, 540–592 (1960)
Lymn, R. W., Taylor, E. W.: Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 10, 4616–4624 (1971)
Mannherz, H. G., Schenk, H., Goody, R. S.: Synthesis of ATP from ADP and inorganic phosphate at the myosin-subfragment 1 active site. Eur. J. Biochem. 48, 287–295 (1974)
Marston, S. B., Tregear, R. T.: Evidence for a complex between myosin and ADP in relaxed muscle fibres. Nature (New Biol.) 235, 23 (1972)
Marston, S. B., Rodger, C. D., Tregear, R. T.: Changes in muscle cross bridges when Β, γ-imido-ATP binds to myosin. J. Mol. Biol. 104, 263–276 (1976)
Mason, P.: Dynamic stiffness and cross bridge action in muscle. Biophys. Struct. Mech. 4, 15–25 (1978)
Paul, R. J., Peterson, J. W.: The mechanochemistry of smooth muscle. In: The biochemistry of smooth muscle. Stephens, N. L. (ed.), pp. 15–39. Baltimore: University Park Press 1977
Podolsky, R. J., Nolan, A. C.: Muscle contraction transients, cross bridge kinetics, and the Fenn effect. Cold Spring Harbor Symp. Quant. Biol. 37, 661–668 (1973)
Pringle, J. W. S.: The mechanical characteristics of insect fibrillar muscle. In: Symposium on insect flight muscle, Oxford 1977. Tregear, R. T. (ed.), pp. 337–344. Amsterdam: Elsevier/North Holland 1977
Pryor, M. G. M.: Mechanical properties of fibres and muscles. Prog. Biophys. 1, 216–268 (1950)
Pybus, J., Tregear, R. T.: Estimates of force and time of actomyosin interaction in an active muscle and of the number interacting at any one time. Cold Spring Harbor Symp. Quant. Biol. 37, 655–660 (1973)
Pybus, J., Tregear, R. T.: The relationship of adenosine triphosphatase activity to tension and power output of insect flight muscle. J. Physiol. 247, 71–89 (1975)
Reedy, M. K.: Ultrastructure of insect flight muscle. I. Screw sense and structural grouping in the rigor cross bridge lattice. J. Mol. Biol. 31, 155–176 (1968)
Reedy, M. K., Holmes, K. C., Tregear, R. T.: Induced changes in orientation of the cross bridges of glycerinated insect flight muscle. Nature 207, 1279 (1965)
Rüegg, J. C., Tregear, R. T.: Mechanical factors affecting the ATPase activity of glycerolextracted insect fibrillar flight muscle. Proc. R. Soc. Ser. B 165, 497–512 (1966)
SchÄdler, M.: Proportionale Aktivierung von ATPase-AktivitÄt und Kontraktionsspannung durch Calziumionen in isolierten contractilen Strukturen verschiedener Muskelarten. Arch. Ges. Physiol. 296, 70–90 (1967)
SchÄdler, M., Steiger, G. J., Rüegg, J. C.: Mechanical activation and isometric oscillation in insect fibrillar muscle. Pflügers Arch. 330, 217–229 (1971)
Schoenberg, M., Wells, J. B., Podolsky, R. J.: Muscle compliance and the longitudinal transmission of mechanical impulses. J. Gen. Physiol. 64, 623–642 (1974)
Steiger, G. J., Rüegg, J. C.: Energetics and “efficiency” in the isolated contractile machinery of an insect fibrillar muscle at various frequencies of oscillation. Pflügers Arch. 307, 1–21 (1969)
Szent-Györgyi, A.: Nature of the contraction of muscle. Nature 167, 380 (1951)
Thorson, J., White, D. C. S.: Distributed representations for actin-myosin interaction in the oscillatory contraction of muscle. Biophysic. J. 9, 360–390 (1969)
Tregear, R. T., Squire, J. M.: Myosin content and filament structure in smooth and striated muscle. J. Mol. Biol. 77, 279–290 (1973)
Trentham, D. R., Eccleston, J. F., Bagshaw, C. R.: Kinetic analysis of ATPase mechanisms. Q. Rev. Biophys. 9, 217–281 (1976)
Truong, X. T.: Viscoelastic wave propagation and rheologic properties of skeletal muscle. Am. J. Physiol. 226, 256–264 (1974)
White, D. C. S.: Links between mechanical and biochemical kinetics of muscle. Cold Spring Harbor Symp. Quant. Biol. 37, 201–213 (1973)
White, D. C. S., Thorson, J.: Phosphate starvation and the nonlinear dynamics of insect fibrillar flight muscle. J. Gen. Physiol. 60, 307–336 (1972)
White, D. C. S., Thorson, J.: The kinetics of muscle contraction. Prog. Biophys. Mol. Biol. 27, 173–255 (1973)
White, D. C. S., Donaldson, M. M. K., Pearce, G. E., Wilson, M. G. A.: The resting elasticity of insect fibrillar flight muscle, and properties of the cross bridge cycle. In: Symposium on insect flight muscle, Oxford 1977. Tregear, R. T. (ed.), pp. 197–208. Amsterdam: Elsevier/North Holland 1977
White, H. D., Taylor, E. W.: Energetics and mechanism of actomyosin adenosine triphosphate. Biochemistry 15, 5818–5826 (1976)
Wiegand, W. B., Snyder, I. W.: A self-energizing pendulum. Trans. Inst. Rubber Ind. 10, 234 (1934)
Wöhlisch, E.: Untersuchungen über elastische, thermodynamische, magnetische und elektrische Eigenschaften tierischer Gewebe. Verh. Physik.-Med. Ges. Würzburg 51, 53–64 (1926)
Woledge, R. C.: The thermoelastic effect of change of tension in active muscle. J. Physiol. 155, 187 (1961)
Yamamoto, T., Herzig, J. W.: Series elastic properties of skinned muscle fibres in contraction and rigor. Pflügers Arch. 373, 21–24 (1978)
Yount, R. G., Babock, D., Ballantyne, W., Ojala, D.: Adenyl imidodiphosphate, an adenosine triphosphate analog containing a P-N-P linkage. Biochemistry 10, 2484–2489 (1971)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kuhn, H.J., Güth, K., Drexler, B. et al. Investigation of the temperature dependence of the cross bridge parameters for attachment, force generation and detachment as deduced from mechano-chemical studies in glycerinated single fibres from the dorsal longitudinal muscle of Lethocerus maximus . Biophys. Struct. Mechanism 6, 1–29 (1979). https://doi.org/10.1007/BF00537592
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00537592