Abstract
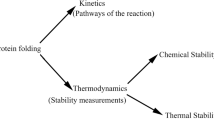
The acquisition of the native three-dimensional structure of proteins consists of sequential folding reactions with well-populated and well-defined structural intermediates. For small proteins successive stages in the folding have been resolved kinetically; these suggest that H-bonded elements of secondary structure are formed first, followed by folding steps to generate the complete tertiary structure.
The rate determining step in the folding of a number of small proteins has been shown to be proline cis ⇌ tram isomerization. As indicated by experiments using fast kinetics the overall folding mechanism, even in a small single-domain molecule like ribonuclease, involves more than one intermediate.
Large protein molecules contain domains which may fold independently. For multi-domain proteins, the pathway of folding therefore involves “folding by parts”, followed by merging of folded domains.
In the case of assembly systems (e.g., oligomeric or multimeric enzymes) folding and association have to be subtly interconnected with respect to the time scale, since the correct assembly of subunits requires their proper folding. In this sense the initial function of oligomeric proteins is their own self-assembly. The corresponding mechanism underlying the spontaneous formation of the native quaternary structure of oligomeric proteins must be the consecutive folding and association of the constituent polypeptide chains.
Equilibrium and kinetic studies have been concerned with a number of dimeric, tetrameric and multimeric enzymes, using enzymatic activity to measure structure formation: alcohol dehydrogenase, aldolase, glyceraldehyde-3-phosphate dehydrogenase, lactic dehydrogenase, malic dehydrogenase, pyruvate dehydrogenase, triose phosphate isomerase, tryptophan synthase.
These experiments make use of the reversibility of protein denaturation, focusing on refolding and reassociation rather than folding and association, because there is no direct approach to structural investigations of the nascent polypeptide chain in vivo.
Optimum conditions of reconstitution yield up to 100% reactivation. After separation of “irreversibly denatured protein”, reconstituted and native protein turn out to be indistinguishable. The major side reaction leading to “wrong aggregation” is due to competition between folding and association.
Due to the high specificity of the association reaction “chimeric” species are not observed, and multimeric systems containing different component enzymes show specific assembly.
The kinetics of reconstitution generally obey an irreversible sequential first- order/second-order mechanism involving inactive monomers; only in the case of aldolase is subunit activity suggested. For a number of oligomeric enzymes renaturation from various denaturants, in the absence or presence of coenzyme is characterized by identical kinetics. For glyceraldehyde-3-phosphate dehydrogenase, however, free NAD as well as a covalently bound NAD-analog are found to enhance the reconstitution.
In the case of assembly structures exceeding the dimer, the observed consecutive folding/association mechanism does not allow us to decide whether the observed second order processes belong to the formation of the dimer or tetramer. Chemical cross-linking and hybridization techniques allow the equilibrium state and the assembly kinetics of oligomeric systems to be analyzed quantitatively. Using this method, e.g., for lactic dehydrogenase, it is obvious that dissociation leads to the homogeneous monomer, while tetramer formation is found to parallel reactivation.
In general, equilibrium and kinetic experiments prove that full enzymatic activity requires association.
In the case of multisubunit enzymes (multienzyme complexes) heterologous interactions of the component enzymes seem to be involved in the rate determining (first order) “reshuffling” processes which generate catalytic activity in the overall enzymatic reaction.
Similar content being viewed by others
References
Adams B, Burgess RJ, Carrey EA, Mackintosh IR, Mitchinson C, Thomas RM, Pain RH (1980) The role of folding units in the kinetic folding of globular proteins. In: Jaenicke R (1980) loc. cit. pp 447–467
Anfinsen CB, Schechter AN, Taniuchi H (1971) Some aspects of the structure of staphylococcal nuclease: Part II. Studies in solution. Cold Spring Harbor Symp. Quant Biol 36:249–255
Anfinsen CB, Scheraga HA (1975) Experimental and theoretical aspects of protein folding. Adv Protein Chem 29:205–300
Baldwin RL (1975) Intermediates in protein folding reactions and the mechanism of protein folding. Annu Rev Biochem 44:453–475
Baldwin RL (1980) The mechanism of folding of RNase A and S. In: Jaenicke R (1980) loc. cit. pp 369–385
Baldwin RL, Creighton TE (1980) Recent experimental work on the pathway and mechanism of protein folding. In: Jaenicke R (1980) loc. cit. pp 217–260
Barksdale AD, Stuehr JE (1972) Kinetics of the helix-coil transition in aqueous poly (L-glutamic acid). J Am Chem Soc 94:3334–3338
Bartholmes P, Jaenicke R (1978) Reassociation and reactivation of yeast GAPDH after dissociation in the presence of ATP. Eur J Biochem 87:563–567
Bernhardt G, Rudolph R, Jaenicke R (1981) Reassociation of LDH from pig heart studied by cross-linking with glutaraldehyde. Z Naturforsch 36c:772–777
Bierzynski A, Kim PS, Baldwin RL (1982) A salt bridge stabilizes the helix formed by the isolated C-peptide of RNase A. Proc Natl Acad Sci USA (in press)
Brandts JF, Brennan M, Lin L-N (1977) Unfolding and refolding occur much faster for a proline-free protein than for most proline-containing proteins. Proc Natl Acad Sci USA 74:4178–4181
Brandts JF, Halvorson HR, Brennan M (1975) Consideration of the possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry 14:4953–4963
Butler PJG, Durham ACH (1977) TMV protein aggregation and the virus assembly. Adv Protein Chem 31:187–251
Chan WW-C (1970) Matrix-bound protein subunits. Biophys Biochem Res Commun 41:1198–1204
Cook RA, Koshland DE Jr (1969) Specificity in the assembly of multisubunit proteins. Proc Natl Acad Sci USA 64:247–254
Creighton TE (1980) Experimental elucidation of pathways of protein unfolding and refolding. In: Jaenicke R (1980) loc. cit. pp 427–446
Creighton TE, Pain RH (1980) Unfolding and refolding of staphylococcus aureus penicillinase by urea-gradient electrophoresis. J Mol Biol 137:431–436
Dautry-Varsat A, Garel J-R (1981) Independent folding regions in AKH dehydrogenase. Biochemistry 20:1396–1401
Garel J-R, Dautry-Varsat A (1980a) The formation of the native structure in the bifunctional enzymes AKH DHI and II from E.coli K 12 and in some of their monofunctional fragments. In: Jaenicke R (1980) loc. cit. pp 485–499
Garel J-R, Dautry-Varsat A (1980b) Sequential folding of a bifunctional allosteric protein. Proc Natl Acad Sci USA 77:3379–3383
Gerschitz J, Rudolph R, Jaenicke R (1978) Refolding and reactivation of L-ADH after dissociation and denaturation in 6 M guanidine · HCl. Eur J Biochem 87:591–599
Girg R, Rudolph R, Jaenicke R (1981) Limited proteolysis of porcine muscle LDH by thermolysin during reconstitution yields dimers. Eur J Biochem 119:301–305
Goldberg ME, Zetina CR (1980) Importance of interdomain interactions in the structure, function and stability of the F1 domains from the Β2 subunit of E. coli TSase. In: Jaenicke R (1980) loc. cit. pp 469–484
Groha C, Bartholmes P, Jaenicke R (1978) Refolding and reactivation of E.coli TSase Β2 subunit after inactivation and dissociation in guanidine. HC1 at acidic pH. Eur J Biochem 92:437–441
Gruenewald B, Nicola CU, Lustig A, Schwarz G, Klump H (1979) Kinetics of the helix-coil transition of a polypeptide with non-ionic side groups, derived from ultrasonic relaxation measurements. Biophys Chem 9:137–147
Hammes GG, Roberts PB (1969) Dynamics of the helix-coil transition in poly-l-ornithine. J Am Chem Soc 91:1812–1816
Hermann R, Jaenicke R, Rudolph R (1981) Analysis of the reconstitution of oligomeric enzymes by cross-linking with glutaraldehyde: Kinetics of reassociation of LDH. Biochemistry 20:5195–5201
Jaenicke R (1974) Reassociation and reactivation of LDH from the unfolded subunits. Eur J Biochem 46:149–155
Jaenicke R (1978) Folding and association of oligomeric enzymes. Naturwissenschaften 65:569–577
Jaenicke R (1980) Protein Folding, Proc 28th Conf German Biochem Soc Regensburg, Sept. 10–12, 1979. Elsevier-North Holland, Amsterdam New York, p 587
Jaenicke R (1981) Enzymes under extremes of physical conditions. Annu Rev Biophys Bioeng 10:1–67
Jaenicke R, Krebs H, Rudolph R, Woenckhaus C (1980) Rate enhancement of reconstitution of GAPDH by covalently bound coenzyme analog. Proc Natl Acad Sci USA 77:1966–1969
Jaenicke R, Lauffer MA (1969) Polymerization-depolymerization of TMV-protein. XII. Further studies on the role of water. Biochemistry 8:3083–3092
Jaenicke R, Perham RN (1982) Reconstitution of PDH from B. stear. Biochemistry (in press)
Jaenicke R, Rudolph R (1980) Folding and association of oligomeric enzymes. In: Jaenicke R (1980) loc. cit. pp 525–548
Jaenicke R, Rudolph R, Heider I (1981a) Specificity in the subunit assembly of oligomeric enzymes: Synchronous reconstitution of mammalian LDH and MDH. Biochem Int 2:23–31
Jaenicke R, Vogel W, Rudolph R (1981b) Dimeric intermediates in the dissociation of LDH. Eur J Biochem 114:525–531
Kim PS, Baldwin RL (1982) Specific intermediates in the folding reactions of small proteins and the mechanism of protein folding. Annu Rev Biochem (in press)
Kellenberger E (1966) Control mechanisms in bacteriophage morphopoiesis. CIBA Foundation Symp, pp 192–228
Labhardt AM (1980) Equilibrium and kinetic stages in the folding of RNase S. In: Jaenicke R (1980) loc. cit. pp 401–425
Labhardt AM, Baldwin RL (1979) Recombination of S-peptide with S-protein during folding of RNase S. J Mol Biol 135:245–254
Lauffer MA (1975) Entropy-driven processes in biology. Springer, Berlin Heidelberg New York, p 264
Levinthal C (1968) Are there pathways for protein folding? J Chim Phys 65:44–45
Levitt M (1980) Computer studies of protein molecules. In: Jaenicke R (1980) loc. cit. pp 17–39
Lin L-N, Brandts JF (1978) Further evidence suggesting that the slow phase in protein unfolding and refolding is due to proline isomerization: A kinetic study of carp parvalbumins. Biochemistry 17:4102–4110
Müller K, Lüdemann H-D, Jaenicke R (1981a) Reconstitution of LDH from pig heart after reversible high-pressure dissociation. Biochemistry 20:5411–5416
Müller K, Lüdemann H-D, Jaenicke R (1981b) Pressure-induced structural changes of pig heart LDH. Biophys Chem 14:101–110
Müller K, Lüdemann H-D, Jaenicke R (1982) Thermodynamics and mechanism of high pressure deactivation and dissociation of porcine LDH. Biophys Chem (in press)
Nall BT, Garel J-R, Baldwin RL (1978) Test of the extended two-state model for the kinetic intermediates observed in the folding transition of RNase A. J Mol Biol 118:317–330
Pfeil W (1981) The problem of the stability of globular proteins. Mol Cell Biochem 40:3–28
Richards FM, Wyckoff HW (1971) Bovine pancreatic ribonuclease. In: Boyer PD (ed) The Enzymes 3rd Ed, Vol 4, pp 647–806
Richardson JS (1981) The anatomy and taxonomy of protein structure. Adv Protein Chem 34:168–339
Rudolph R, Gerschitz J, Jaenicke R (1978) Effect of Zn (II) on the refolding and reactivation of L-ADH. Eur J Biochem 87:601–606
Rudolph R, Zettlmei\l G, Jaenicke R (1979) Reconstitution of LDH. Noncovalent aggregation vs reactivation. 2. Reactivation of irreversibly denatured aggregates. Biochemistry 18:5572–5575
Schade BC, Lüdemann H-D, Rudolph R, Jaenicke R (1980) Kinetics of reconstitution of porcine muscle LDH after reversible high-pressure dissociation. Biophys Chem 11:257–263
Schade BC, Rudolph R, Lüdemann H-D, Jaenicke R (1980) Reversible high-pressure dissociation of LDH from pig muscle. Biochemistry 19:1121–1126
Schmid FX, Baldwin RL (1978) Acid catalysis of the formation of the slow folding species of RNase A: Evidence that the reaction is proline isomerization. Proc Natl Acad Sci USA 75:4764–4768
Schmid FX, Baldwin RL (1979) Detection of an early intermediate in the folding of RNase A by protection of amide protons against exchange. J Mol Biol 135:199–215
Schulz GE, Schirmer HR (1979) Principles of protein structure. Springer, New York Heidelberg Berlin, p 314
Seifert T, Bartholmes P, Jaenicke R (1982) Reconstitution of the isolated Β 2 subunits of TSase from E. coli after dissociation induced by high hydrostatic pressure: Equilibrium and kinetic studies. Biophys Chem 15:1–8
Stadtman ER (1966) Allosteric regulation of enzyme activity. Adv Enzymol 28:41–154
Sturtevant JM (1977) Heat capacity and entropy changes in processes involving proteins. Proc Natl Acad Sci USA 74:2236–2240
Sturtevant JM, Velicelebi G, Jaenicke R, Lauffer MA (1981) Scanning calorimetric investigation of the polymerization of the coat protein of TMV. Biochemistry 20:3792–3800
Tanford C (1968) Protein denaturation. Adv Protein Chem 23:122–282
Tanford C (1970) Protein denaturation. Adv Protein Chem 24:1–95
Teipel JW, Koshland DE Jr (1971) Kinetic aspects of conformational changes in proteins. Biochemistry 10:792–805
Thomas KA, Schechter AN (1980) Protein folding: Evolutionary, structural and chemical aspects. In: Goldberger RF (ed) Biological regulation and development, vol 2. Plenum Press, New York London, pp 43–100
Wetlaufer DB (1980) Practical consequences of protein folding mechanisms. In: Jaenicke R (1980) loc. cit. pp 323–329
Wetlaufer DB (1981) Folding of protein fragments. Adv Protein Chem 34:61–92
Wetlaufer DB, Ristow S (1973) Acquisition of three-dimensional structure of proteins. Annu Rev Biochem 42:135–158
Zabori S, Rudolph R, Jaenicke R (1980) Folding and association of triose phosphate isomerase from rabbit muscle. Z Naturforsch 35c:999–1004
Zettlmei\l G, Rudolph R, Jaenicke R (1979a) Reconstitution of LDH: Non-covalent aggregation vs reactivation. I. Physical properties and kinetics of aggregation. Biochemistry 18:5567–5571
Zettlmei\l G, Rudolph R, Jaenicke R (1979b) Effects of low concentrations of guanidine · HCl on the reconstitution of LDH from pig muscle in vitro. Eur J Biochem 100:593–598
Zettlmei\l G, Rudolph R, Jaenicke R (1981) Reconstitution of LDH after acid dissociation: The yield of reactivation is determined by conformational rearrangements of the dissociated monomers. Eur J Biochem 121:169–175
Zettlmei\l G, Rudolph R, Jaenicke R (1982) Reconstitution of LDH: The ratio of reactivation to aggregation is not determined by cis ⇌ trans isomerization of X-proline peptide bonds. Eur J Biochem (in press)
Author information
Authors and Affiliations
Additional information
Dedicated to Professor Ernst M. Helmreich on the occasion of his sixtieth birthday
Rights and permissions
About this article
Cite this article
Jaenicke, R. Folding and association of proteins. Biophys. Struct. Mechanism 8, 231–256 (1982). https://doi.org/10.1007/BF00537204
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00537204