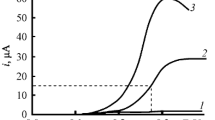
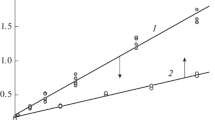
Abstract
In the primary process of electrochemical reduction of substituted 3-nitropyridines in dimethylformamide, their anion radicals are formed. This also takes place in the reduction of 3,5-dinitropyridines and 3-nitropyridines with a nitrophenyl substituent at position 2 or 4. For these dinitro derivatives, however, secondary free radicals are formed as well; in a basic medium, these are the products of reduction of the corresponding Meisenheimer complexes. Serving as the reaction center for electroreduction is the nitro group on the pyridine ring, not the group on the phenyl ring. For the mononitropyridines and dinitropyridines that were studied, free radicals of the nitropyridine type are formed as the primary species. The structure of the primary and secondary free radicals was established by analysis of the hyperfine structure of their ESR spectra.
Similar content being viewed by others
References
Landolt-Börnstein, Zahlenwerte und Funktionen aus Naturwissenschaften und Technik. Neue Serie, Gesamtherausgeber K.-H. Hellwege, Springer Verlag, Berlin (1980), Group II, Vol. 9, No. d1, p. 904.
R. A. Gavar, Ya. P. Stradyn', L. Kh. Baumane, and K. Enish, Khim. Geterotsikl. Soedin., No. 12, 1631 (1991).
M. Trushule, É. Kupche, I. Augustane, V. M. Verovskii, É. Lukevits, L. Baumane, R. Gavar, and Ya. Stradyn', Khim. Geterotsikl. Soedin., No. 12, 1687 (1991).
L. Kh. Baumane, Ya. P. Stradyn', R. A. Gavar, B. S. Chekavichus, and G. Ya. Dubur, Khim. Geterotsikl. Soedin., No. 4, 481 (1991).
L. Baumane, J. Stradins, R. Gavars, and J. Duburs, Electrochim. Acta, 32, 2599 (1992).
Ya. Stradyn', L. Baumane, R. Gavars, B. Chekavichus, and G. Duburs, Khim. Geterotsikl. Soedin., No. 11, 1498 (1992).
A. J. Bard (editor), Encyclopedia of Electrochemistry of the Elements, Organic Section, Marcel Dekker, New York (1979), Vol. 13, p. 370.
C. Hansch, A. Leo, and R. W. Taft, Chem. Rev., 91, 165 (1991).
V. L. Rusinov and O. N. Chupakhin, Nitroazines [in Russian], Nauka, Novosibirsk (1991).
A. P. Chatrousse, F. Terrier, and R. Schall, J. Chem. Res., Synop., 9, 228 (1977).
V. F. Starichenko, V. P. Sokolov, and S. M. Shein, Izv. Akad. Nauk SSSR, Ser. Khim., No. 8, 1839 (1971).
Yu. M. Kargin, V. V. Kondranina, and N. I. Semakhina, Izv. Akad. Nauk SSSR, Ser. Khim., No. 2, 278 (1971).
Ya. P. Stradyn', R. A. Gavar, and L. Kh. Baumane, Izv. Akad. Nauk Latv. SSR, No. 2 (463), 73 (1986).
L. H. Piette, P. Ludwig, and R. N. Adams, Anal. Chem., 8, 916 (1962).
B. Vigante, Ya. Ozols, and G. Duburs, Khim. Geterotsikl. Soedin., No. 1, 64 (1993).
G. Franckowiak, H. Böshagen, F. Bossert, S. Goldman, H. Meyer, E. Weniger, J. Stoltefys, M. Schram, G. Thomas, and R. Towart, FRG Pat. 3,206,671 [Chem. Abstr., 98, 215488 (1983)].
A. Krowczynski and L. Kozerski, Synthesis, No. 6, 489 (1983).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1079–1087, August, 1993.
Rights and permissions
About this article
Cite this article
Stradyn', Y., Gavars, R., Baumane, L. et al. Free radicals in the electrochemical reduction of certain mononitro and dinitro derivatives of pyridine. Chem Heterocycl Compd 29, 918–925 (1993). https://doi.org/10.1007/BF00534270
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00534270