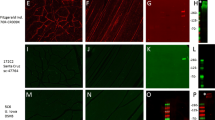
Summary
This paper describes two new monoclonal antibodies reactive with human specific type IV collagen epitopes in frozen as well as routinely fixed and processed tissue sections. The antibodies (1042 and 1043) were raised against human placental type IV collagen and were shown by immunoblotting and ELISA tests to react exclusively with type IV collagen determinants. Extensive immunohistochemical survey studies on panels of tissues from various species, using unfixed cryosat sections, demonstrated that antibody 1042 reacted only with human type IV collagen whereas antibody 1043 in addition reacted with rabbit type IV collagen. All tissues showed homogeneous staining of the basement membrane, indicating that the detected epitopes did not show organ-specific distribution.
Tissue processing protocols for using these monoclonal antibodies on routinely processed paraffin embedded tissues were developed. It was found that whereas polyclonal antitype IV collagen antisera required pepsin digestion, our monoclonal antibodies required pronase or papain digestion to restore type IV collagen immunoreactivity in paraffin sections.
It is concluded that these monoclonal anti-type IV collagen antibodies detect species specific epitopes which can be detected in routinely processed paraffin embedded tissues after appropriate enzyme pretreatment.
Similar content being viewed by others
References
Albrechtsen R, Nielsen M, Wever U, Engvall E, Ruoslahti E (1981) Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res 41:5076–5081
Barsky SH, Siegal GP, Janotta F, Liotta LA (1983) Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab Invest 49:140–147
Barsky SH, Rao NC, Restrepo C, Liotta LA (1984) Immunocytochemical enhancement of basement membrane antigens by pepsin: applications in diagnostic pathology. Am J Clin Pathol 82:191–194
Bosman FT, Havenith MG, Cleutjens JPM (1985) Basement membranes in cancer. Ultrastruct Pathol 8:291–304
Crouch E, Sage H, Bornstein P (1980) Structural basis for apparent heterogeneity of collagens in human basement membranes: type IV procollagen contains two distinct chains. Proc Natl Acad Sci USA 77:745–749
Damjanov I, Damjanov N, Knowles BB, Engvall E (1985) Origin of laminin in the extracellular matrix of human tumor xenografts in nude mice. Virchows Arch Cell Pathol 49:45–52
Foellmer HG, Madri JA, Furthmayr H (1983) Monoclonal antibodies to type IV collagen: probes for the study of structure and function of basement membranes. Lab Invest 48:639–649
Hay ED (1978) Role of basement membranes in development and differentiation. In: Kefalides NA (ed) Biology and chemistry of basement membranes. Academic Press, New York, pp 119–136
Holmstrup P (1985) Studies on the deposition of laminin and type IV collagen in human oral mucosa transplanted to nude mice. Acta Pathol Microbiol Immunol Scand Sect A 93:1–8
Kanwar YS, Farquhar MG (1979) Amnionic sites in the glomerular basement membrane. In vivo and vitro localization to the laminae rarae by cationic probes. J Cell Biol 81:137–153
Kefalides NA, Alper R, Clark C (1979) Biochemistry and metabolism of basement membranes. Int Rev Cytol 61:167–228
Kühn K, Wiedemann H, Timpl R, Risteli J, Dieringer H, Voss T, Glanville RW (1981) Macromolecular structure of basement membrane collagens. Identification of 7S collagen as a cross-binding domain of type IV collagen. FEBS Lett 125:123–128
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227:680–685
Martinez-Hernandez A, Amenta PS (1983) The basement membrane in pathology. Lab Invest 48:656–677
Miettinen M, Foidart JH, Ekblom P (1983) Immunohistochemical demonstration of laminin, the major glycoprotein of basement membranes, as an aid in the diagnosis of soft tissue tumors. Am J Clin Pathol 79:306–311
Miller EJ, Kent Rhodes R (1982) Preparation and characterization of the different types of collagen. Methods in enzymology, vol 82. Academic Press, New York, pp 32–64
Mynderse LA, Hassel J, Kleinman HK, Martin GR, Martinez-Hernandez A (1983) Loss of heparan sulphate proteoglycan from glomerular basement membrane of nephrotic rats. Lab Invest 48:292–302
Odermatt BF, Lang AB, Ruttner JR, Winterhalter KH, Trueb B (1984) Monoclonal antibodies to human type IV collagen: useful reagents to demonstrate the heterotrimeric nature of the molecule. Proc Natl Acad Sci USA 81:7343–7347
Ogawa K, Oguchi M, Yamabe H, Nakashima Y, Hamashima Y (1986) Distribution of collagen type IV in soft tissue tumors. Cancer 58:269–277
Sakai LY, Engvall E, Hollister DW, Burgeson RE (1982) Production and characterization of a monoclonal antibody to human type IV collagen. Am J Pathol 108:310–318
Sanders EJ (1983) Recent progress towards understanding the roles of the basement membrane in development. Can J Biochem Cell Biol 61:949–956
Scheinman JI, Tsai C (1984) Monoclonal antibody to type IV collagen with selective basement membrane localization. Lab Invest 50:101–112
Sundarraj N, Willson J (1982) Monoclonal antibody to human basement membrane collagen type IV. Immunology 47:133–140
Timpl R, Wiedemann H, Van Delden V, Furthmayer H, Kühn K (1981) A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem 12:203–211
Towbin H, Stachelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Vracko R (1974) Basal lamina scaffold: anatomy and significance for maintainance of orderly tissue structure. Am J Pathol 77:314–338
Woodley DT, O'Keefe EJ, Reese MJ, Mechanic GH, Briggaman RA, Gammon WR (1986) Epidermolysis bullosa acquisita antigen, a new major component of the cutaneous basement membrane, is a glycoprotein with collagenous domains. J Invest Dermatol 86:668–672
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Havenith, M.G., Cleutjens, J.P.M., Beek, C. et al. Human specific anti-type IV collagen monoclonal antibodies, characterization and immunohistochemical application. Histochemistry 87, 123–128 (1987). https://doi.org/10.1007/BF00533396
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00533396