Abstract
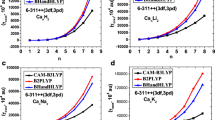
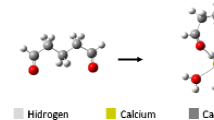
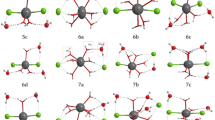
Ab initio SCF calculations have been carried out for calcium dication surrounded by 1–4 or 6 water molecules using several basis sets. The structure of the hydrated ions is examined and the hydration energy estimated. The changes in the structure of water within the complex are qualitatively explained in terms of charge transfer and coulombic interactions among the atoms. Finally, the influence of electron correlations is discussed.
Similar content being viewed by others
References and notes
See for instance: Bernal JD, Fowler TH (1933) J Chem Phys 1:515, 548
The importance of Ca2+ in the mitochondrial exchange mechanism is well stated, as an hydrated entity, see for instance: Crafoli E (1977) In: Allen MJ, Masué JP (eds) Living systems as energy converters. North-Holland, Amsterdam
Bockris JOM, Reddy O (1972) Modern electrochemistry, vol. 1. Wiley, New York
Dzidic I, Kebarle P (1970) J Chem Phys 74:1466
Kollmann PA, Kuntz ID (1972) J Am Chem Soc 94:9236; Clementi E, Popkie H (1972) J Chem Phys 57:1077; Clementi E (1980) Computational aspects for large chemical systems (Lect Notes Chem, vol. 19) Springer, Berlin Heidelberg New York
Corongiu G, Clementi E (1978) J Chem Phys 69:4885
Hermanssonn K, Olavson I (1984); Theor Chim Acta 64:265; Lischka H (1979) Appl Chem 51:1627
Dupuis M, Rys J, King HF (1976) J Chem Phys 65:111
Poirier R, Peterson M (1984) University of Toronto
Tatewaki H, Huzinaga S (1980) J Comp Chem 1:205; Sakai Y, Tatewaki H, Huzinaga S (1981) J Comp Chem 2:100
Hobza P, Sauer J (1984) Theor Chim Acta 65:279; Sauer J, Hobza P (1984) Theor Chim Acta 65:291
Clementi E (1974) Coloquio Internacional de Quimicos Teoricos de Expresion Latina. IBM Research Laboratories, San Jose
Wachter AJH (1970) J Chem Phys 52:1033, 1036
Dunning TH Jr (1970) J Chem Phys 53:2823; Dunning TH Jr, Hay PJ (1977) In: Dunning TH Jr (ed) Modern theoretical chemistry, vol 2. Plenum Press, New York
Boys SF, Bernardi F (1970) Mol Phys 19:553
Johanson A, Kollmann PA, Rotherberg S (1973) Theor Chim Acta 29:163
Schwencke DW, Truhlar DG (1985) J Chem Phys 82:2418; (1986) ibid 84:4113; (1987) ibid 86:3760
Carsky P, Hess BA Jr, Schaad LJ (1984) J Comp Chem 5:280
Arbman M, Siegbahn H, Petersson L, Siegbahn P (1985) Mol Phys 54:1149
Hashimoto K, Yoda N, Iwata S (1987) Chem Phys 116:193
Mulliken RS, Pearson WB (1969) Molecular complexes. Wiley, New York
del Re G, Otto P, Ladik J (1980) Isr J Chem 19:265
Gutmann V (1978) The donor acceptor approach to molecular interactions. Plenum Press, New York
Hehre WJ, Radom L, Schleyer PvR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cachau, R.E., Villar, H.O. & Castro, E.A. Theoretical study of the calcium dication hydrates. Theoret. Chim. Acta 75, 299–306 (1989). https://doi.org/10.1007/BF00533195
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00533195