Abstract
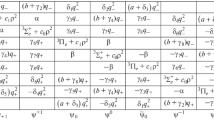
The influence of vibronic coupling on the average paramagnetism and the paramagnetic anisotropy of a cubic complex, the electronic ground state 2T2 of which is perturbed by a trigonal field, is investigated. It is necessary to introduce the following parameters: the spin-orbit coupling coefficient λ, the vibronic coupling coefficient x, the frequency ħω g3 of the E modes of vibration, the splitting Δ of the 2 T 2 level in the trigonal field and the covalence parameter k.
For given x and \(\varrho \left( { = \frac{{3\lambda }}{{2h\omega _\varepsilon }}} \right)\), the influence of the vibronic coupling is more important if in the trigonal field the electronic ground state of the complex is 2E than if it is 2A. For given x and v (=Δ/λ), the smaller ¦ρ¦, the greater the influence of vibronic coupling. The respective effects of vibronic coupling and covalence are compared. Finally, the case of the first row transition-metal complexes is briefly discussed.
Similar content being viewed by others
References
Kahn,O., Kettle,S.F.A.: Theoret. chim. Acta (Berl.) 27, 187 (1972).
Van Vleck,J. H.: The theory of electronic and magnetic susceptibilities. Oxford University Press (1932).
Sturge, M.D.: Solid State Physics 91 (1967).
Kahn,O., Kettle,S.F.A.: Molecular Physics à paraître.
Gladney,H.M., Swalen,J.D.: J. chem. Physics 42, 1999 (1965).
Dutta-Roy,K.S., Chakravarty,A.S., Buse,A.: Indian J. Physics Proc. Indian Assoc. Cultivat. Sci. 38, 483 (1959).
Bleaney,B., Bogle,G.S., Cooke,A.H., Duffus,R.J., O'Brien,M.C.M., Stevens,K.W.H.: Proc. Physics Soc. 268, 57 (1955).
Van Vleck,J.H.: J. chem. Physics 7, 61 (1939).
Figgis,B.N.: Trans. Faraday Soc. 57, 198 et 204 (1961).
Gerloch, M.: J. chem.Soc. 1968, 2023.
Kamimura,H., Mizuhashi,S.: J. appl. Physics 39, 684 (1968).
Mizuhashi,S.: J. physic. Soc. Japan 26, 468 (1969).
Sasaki,K., Obata,Y.: J. physic. Soc. Japan 28, 1157 (1970).
Dunn,T.M.: Trans. Faraday Soc. 57, 1441 (1961).
Hass,H., Sheline,R.K.: J. Amer. chem. Soc. 88, 3219 (1966).
Nassif,P.J., Couch,T.W., Hatfield,W.E., Villa,J.F.: Inorg. Chemistry 10, 368 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kahn, O., Kettle, S.F.A. Couplage vibronique et anisotropie paramagnétique dans un complexe cubique 2 T 2 soumis à un champ trigonal. Theoret. Chim. Acta 29, 359–374 (1973). https://doi.org/10.1007/BF00532191
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00532191