Abstract
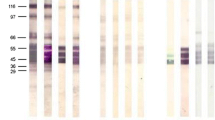
The technique of SDS-polyacrylamide gel electrophoresis and immunoblotting was used to study the evolution of the IgG antibody response to Toxoplasma gondii antigens in sequential sera of acutely infected patients. The results show that the IgG immunoblot pattern can be a useful marker for the stage of T. gondii infection. A 35-kD antigen elicited the first IgG response soon after exposure. During the course of infection additional bands appeared consecutively, following a constant sequence, to evolve to a late-stage-specific pattern with about 13–15 major bands. This pattern, which was reached at least 4 months after infection, was also found in the immunoblots of 28 patients with a chronic (latent) T. gondii infection.
Similar content being viewed by others
References
Araujo FG, Remington JS (1984) Partially purified antigen preparations of Toxoplasma gondii protect against lethal infection in mice. Infect Immun 45:122–126
Blake MS, Johnston KH, Russell-Jones GL, Gotschlich EC (1984) A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on western blots. Anal Biochem 136:175–179
Braveny I, Winter W, Disko R (1978) A method of mass cultivation of Toxoplasma gondii in cell culture. Tropenmed Parasitol 29:432–434
Erlich HA, Rodgers G, Vaillancourt P, Araujo FG, Remington JS (1983) Identification of an antigen-specific immunoglobulin M antibody associated with acute Toxoplasma infection. Infect Immun 41:683–690
Handman E, Goding JW, Remington JS (1980) Detection and characterization of membrane antigens of Toxoplasma gondii. J Immunol 124:2578–2583
Johnson AM, McDonald PJ, Neoh SH (1983) Molecular weight analysis of soluble antigens from Toxoplasma gondii. J Parasitol 69:459–464
Kasper LH, Crabb JH, Pfefferkorn ER (1983) Purification of a major membrane protein of Toxoplasma gondii by immunoabsorption with a monoclonal antibody. J Immunol 130:2407–2412
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lindenschmidt EG (1986) Demonstration of immunoglobulin M class antibodies to Toxoplasma gondii antigenic component p 35000 by enzyme-linked antigen immunosorbent assay. J Clin Microbiol 24:1045–1049
Naot Y, Remington JS (1980) An enzyme-linked immunosorbent assay for detection of IgM antibodies to Toxoplasma gondii: use for diagnosis of acute acquired toxoplasmosis. J Infect Dis 142:757–766
Naot Y, Guptill DR, Remington JS (1982) Duration of IgM antibodies to Toxoplasma gondii after acute acquired toxoplasmosis. J Infect Dis 145:770
Naot Y, Guptill DR, Mullenax J, Remington JS (1983) Characterization of Toxoplasma gondii antigens that react with human immunoglobulin M and immunoglobulin G antibodies. Infect Immun 41:331–338
Partanen P, Turunen HJ, Paasivuo R, Forsblom E, Suni J, Leinikki PO (1983) Identification of antigenic components of Toxoplasma gondii by an immunoblotting technique. FEBS Lett 158:252–254
Partanen P, Turunen HJ, Paasivuo R, Leinikki PO (1984) Immunoblot analysis of Toxoplasma gondii antigens by human immunoglobulin G, M and A antibodies at different stages of infection. J Clin Microbiol 20:133–135
Potasman I, Araujo FG, Desmonts G, Remington JS (1986a) Analysis of Toxoplasma gondii antigens recognized by human sera obtained before and after acute infection. J Infect Dis 154:650–657
Potasman I, Araujo FG, Remington JS (1986b) Toxoplasma antigens recognized by naturally occurring human antibodies. J Clin Microbiol 24:1050–1054
Santoro F, Afchain D, Pierce R, Cesbron YJ, Ovlaque G, Capron A (1985) Serodiagnosis of Toxoplasma infection using a purified parasite protein (P30). Clin Exp Immunol 62:262–269
Sharma SD, Mullenax J, Araujo FG, Erlich HA, Remington JS (1983) Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J Immunol 131:977–983
Thulliez P, Remington JS, Santoro F, Ovlaque G, Sharma S, Desmonts G (1986) Une nouvelle réaction d'agglutination pour le stade évolutif de la toxoplasmose acquise. Pathol Biol 34:173–177
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Verhofstede C, Sabbe L, Van Renterghem L (1987) Ability of the enzyme linked immunosorbent assay to detect early immunoglobulin G antibodies to Toxoplasma gondii. Eur J Clin Microbiol 6:147–151
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Verhofstede, C., Van Gelder, P. & Rabaey, M. The infection-stage-related IgG response to Toxoplasma gondii studied by immunoblotting. Parasitol Res 74, 516–520 (1988). https://doi.org/10.1007/BF00531628
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00531628