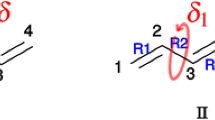
Abstract
Two methods are proposed for dealing with a three-membered ring which is incorporated into a large molecule. The first, an all-valence electron approach, is an iterative CNDO/2 which provides, through bond indices, self-consistent hybridizations and valency angles. The second is compatible with π-electron approaches and aims at the study of conjugation and transmission of conjugation. The methods are illustrated by application to the unsubstituted cyclopropane molecule.
Résumé
On propose deux méthodes pour étudier les molécules contenant une composante cyclopropanique. La première, qui tient compte de tous les électrons de valence, est une CNDO/2 itérative qui fournit — par l'intermédiaire des indices carrés de liaison — des valeurs auto-cohérentes pour l'hybridation et les angles de valence. L'autre, compatible avec les techniques π-électroniques, a pour but l'étude de la conjugaison et de la transmission de conjugaison. Les méthodes sont illustrées par application au cyclopropane non-substitué.
Zusammenfassung
Es werden zwei Methoden zur Behandlung von Molekülen, die einen Dreiring enthalten, vorgeschlagen. Die eine besteht in der iterativen Anwendung des CNDO/2-Verfahrens mit selbst-konsistenten Hybridisierungen und Valenzwinkeln. Die zweite entspricht etwa einem π-Elektronenverfahren und dient besonders dem Studium von Konjugationseffekten. Illustriert werden beide am Beispiel des einfachen Cyclopropan.
Similar content being viewed by others
References
Meyer,A.Y., Kesten,Y.: Theoret. chim. Acta (Berl.) 20, 352 (1971).
Robinson,R.: J. chem. Soc. (London) 109, 1042 (1916).
Pète,J.-P.: Bull. Soc. chim. France 1967, 357.
Pews,R.G., Ojha,N.D.: J. Amer. chem. Soc. 91, 5769 (1969).
Smith,M.: In: Coffey,S. (ed.): Rodd's chemistry of carbon compounds, 2nd. Ed., Vol. 2, p. 19 et seq. Amsterdam: Elsevier 1967.
Rogers,M.T., Roberts,J.D.: J. Amer. chem. Soc. 68, 843 (1946).
Streitwieser,A., cited in Kosower,E.M.: An introduction to physical organic chemistry, p. 30. New York: Wiley 1968.
Wagner,P., Duncan,A.B.F.: J. chem. Physics 21, 516 (1953).
Raymonda,J.W., Simpson,W.T.: J. chem. Physics 47, 430 (1967).
Mårtensson,O., Sperber,G.: Acta chem. Scand. 24, 1749 (1970).
Brown,R.D, Krishna,V.G.: J. chem. Physics 45, 1482 (1966).
Music,J.F., Matsen,F.A.: J. Amer. chem. Soc. 72, 5256 (1950).
Clark,D.T.: Theoret. chim. Acta (Berl.) 10, 111 (1968).
Hoffmann, R.: J. chem. Physics 40, 2480 (1964).
Yonezawa,T., Shimizu,K., Kato,H.: Bull. chem. Soc. Japan 40, 456 (1967).
Buenker,R.J., Peyerimoff,S.D.: J. physic. Chem. 73, 1299 (1969).
Kochanski,E., Lehn,J.-M.: Theoret. chim. Acta (Berl.) 14, 281 (1969).
Basch,H., Robin,M.B., Kuebler,W.A, Baker,C., Turner,D.W.: J. chem. Physics 51, 52 (1969).
Preuss,H., Diercksen,G.: Int. J. quant. Chemistry 1, 361 (1967).
Bonaccorsi,R., Scrocco,E., Tomasi,J.: J. chem. Physics 52, 5270 (1970).
Bernett,W.A.: J. chem. Educ. 44, 17 (1967).
Sugden,T.M.: Nature 160, 367 (1947).
Walsh,D.D.: Trans. Faraday Soc. 45, 179 (1949).
Kilpatrick,J.E., Spitzer,R.: J. chem. Physics 14, 463 (1946).
Coulson,C.A., Moffitt,W.E.: Philos. Mag. 40, 1 (1949).
Wiberg,K.: Tetrahedron 24, 1083 (1968).
Gordon,M.S.: J. Amer. chem. Soc. 91, 3122 (1969).
Segal,G.A.: J. chem. Physics 47, 1876 (1967).
Berthier,G., Meyer,A.Y., Praud,L.: Proc. 3rd Jerusalem Symposium on quantum chemistry and biochemistry, p. 174. Jerusalem: Israel Academy 1971.
Meyer,A.Y., Serre,J.: Theoret. chim. Acta (Berl.) 8, 117 (1967).
—: J. Chim. physique 65, 837 (1968).
—: Theoret. chim. Acta (Berl.) 9, 401 (1968).
Parr,R.G.: The quantum theory of molecular electronic structure. New York: Benjamin 1963.
Diehl,P., Khetrapal,C.L., in Diehl,P., Fluck,E., Kosfeld,R.: NMR-basic principles and progress, Vol. 1, p. 62. Berlin: Springer-Verlag 1969.
Bastiansen,O.: Acta crystallogr. 17, 538 (1964).
Coulson,C.A., Goodwin,T.H.: J. chem. Soc. (London) 1962, 2851.
Pople,J.A., Segal,G.A.: J. chem. Physics 44, 3289 (1966). Computer programm, Clark,P.A., Ragle,J.A.: CNDOTWO-SCF-LCAO-MO, QCPE 100.
Coulson, C.A.: Valence, 2nd Ed., reprinted with further corrections. Oxford University Press, 1969.
Trindle,C., Sinanolu,O.: J. Amer. chem. Soc. 91, 853 (1969).
Salem,L.: Molecular orbital theory of conjugated systems. New York: Wiley 1966.
Trindle,C.: J. Amer. chem. Soc. 91, 219 (1969).
Veillard,A., Del Re,G.: Theoret. chim. Acta (Berl.) 2, 55 (1964).
Fritchie,C. J.: Acta crystallogr. 20, 27 (1966).
Hartman,A., Hirschfeld,F.L.: Acta crystallogr. 20, 80 (1966).
Murrell,J.N.: The theory of the electronic spectra of organic molecules. London: Methuen 1963.
Roothaan,C.C.J.: J. chem. Physics 19, 1445 (1951).
Mulliken,R.S., Rieke,C.A., Orloff,D., Orloff,H.: J. chem. Physics 17, 1248 (1949).
Roothaan,C.C.J.: Rev. mod. Physics 23, 69 (1951).
Fischer-Hjalmars,I.: Advances quant. Chem. 2, 25 (1965).
Herzfeld,F.K.: Chem. Rev. 41, 233 (1947).
Author information
Authors and Affiliations
Additional information
For Part V, see Ref. [1].
Rights and permissions
About this article
Cite this article
Meyer, A.Y. Multi-conformational compounds with two absorbing groups. Theoret. Chim. Acta 22, 271–282 (1971). https://doi.org/10.1007/BF00530272
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00530272