Abstract
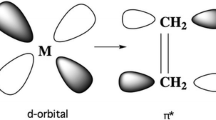
CASSCF and CCI calculations have been performed to analyze the bonding in Ni(C2H4)2. Three different relative orientations of the two olefins have been studied. It is found that a structure with D2d symmetry, where the C-C bonds in the two olefins make a 90 degree angle to each other, gives the lowest energy. A D2h form, with the two C-C bonds and Ni in the same plane, is 10.3 kcal/ mol higher in energy. The reason for the preference of the D2d form is analyzed in terms of valence bond theory, and is found to be due to a d 8 structure with two simultaneous d π bonds. A C 2v form, for which the two nickel olefin bonds make a 103 degree angle to each other and the C-C bonds are parallel to each other, is 32 kcal/mol higher in energy than the D2d form. The low binding energy of the C 2v form is due to a poor σ interaction with inefficient sd hybridization.
Similar content being viewed by others
References
Dewar MJS (1951) Bull Soc Chim Fr 18:C71
Chatt J, Duncanson LA (1953) J Chem Soc 2939
Walch SP, Goddard III WA (1976) J Am Chem Soc 98:7908
Rives AB, Fenske RF (1981) J Chem Phys 75:1293
Bagus PS, Roos BO, (1981) J Chem Phys 75:5961
Bauschlicher CW, Bagus PS, (1984) J Chem Phys 81:5889
Blomberg MRA, Brandemark U, Siegbahn PEM, Broch-Mathisen K, Karlström G (1985) J Phys Chem 89:2171
Ozin GA, Power WJ, Upton TH, Goddard III WA (1978) J Am Chem Soc 100:4750; Upton TH, Goddard III WA (1978) J Am Chem Soc 100:321
Åkermark B, Almemark M, Almlöf J, Bäckvall J-E, Roos B, Stögård Å (1977) J Am Chem Soc 99:4617
Widmark P-O, Roos BO, Siegbahn PEM (1985) J Phys Chem 89:2180
Rösch N, Hoffmann R (1974) Inorg Chem 13:2656
Pitzer RM, Schaefer III HF (1979) J Am Chem Soc 101:7176
Siegbahn PEM (1985) J Am Chem Soc 107:7408
Pearson RG (1976) Symmetry rules for chemical reactions. Wiley, New York
Grubbs RH, Miyashita A, Liu M-IM, Burk PL (1977) J Am Chem Soc 99:3863
Tatewaki H, Huzinaga S (1979) J Chem Phys 71:4339
Pettersson L, Wahlgren U (1982) Chem Phys 69:185
Hay PJ (1977) J Chem Phys 66:4337
Tatewaki H, Huzinaga S (1980) J Comput Chem 1:205
Huzinaga S (1965) J Chem Phys 42:1293
Brandemark U, Siegbahn PEM: to be published
Roos BO, Taylor PR, Siegbahn PEM (1980) Chem Phys 48:157
Siegbahn PEM (1983) Int J Quantum Chem 23:1869
Blomberg MRA, Siegbahn PEM (1983) J Chem Phys 78:5682
Davidson ER (1974) In: Daudel R, Pullmann B (eds) The world of quantum chemistry. Reidel, Dordrecht
Stevens AE, Feigerle CS, Lineberger WC (1982) J Am Chem Soc 104:5026
Fischer K, Jonas K, Wilke G (1973) Angew Chem Int Ed Engl 12:565
Moore CE (1952) Atomic energy levels. Natl Bur Std, Washington
Blomberg MRA, Siegbahn PEM (1984) Chem Phys 87:189
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Siegbahn, P.E.M., Brandemark, U.B. Orientational preference of two ethylene ligands bound to a nickel atom. Theoret. Chim. Acta 69, 119–133 (1986). https://doi.org/10.1007/BF00527684
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00527684